Question: You have been assigned to simulate a flash evaporator that separates a Liquid feed stream containing benzene and toluene at temperature T F (?C) into
You have been assigned to simulate a flash evaporator that separates a Liquid feed stream containing benzene and toluene at temperature TF (?C) into liquid and vapor product streams in equilibrium at temperature T(?C) and pressure P(mm Hg). The compositions of the product streams are related by Raoults law (Equation 6.4-1), and the component vapor pressures are expressed by the Antoine equation (Table B.4).
A spreadsheet that performs the required material and energy balances and vapor?liquid equilibrium calculations on this process unit are shown on the next page. In the test case, a 40 mole% benzene-60 mole% toluene mixture is fed to the evaporator at TF = 120?C and a pressure high enough to assure that the feed stream remains in the liquid state. The unit operates at T = 100?C and P = 800 mm Hg. The heat capacities of liquid benzene and toluene have been taken to be 0.138 kJ/ (mol??C) and 0.165 kJ/(mol??C). respectively and the vapor heat capacities and heats of vaporization of both species are those given in Appendix B.
(a) Derive expressions for the quantities in boldface on the spreadsheet-that is, the flow rates and compositions of the liquid and vapor product streams, the vapor pressures of ben.ze3e and toluene at the evaporator temperature, the fractional vaporizations of benzene and toluene the specific enthalpies of benzene and toluene liquid at TF and T and of benzene and toluene vapor at T (all relative to the liquid species at 0?C). and the required rate of heat transfer to me evaporator.
(b) Create a spreadsheet that replicates the one shown above, entering the formula derived in part (a) and, if possible, imbedding the graphics for the streams and the proc unit In the formulas, enter the cell addresses for variables appearing elsewhere on the spreadsheet: for example, if a formula involves the mole fraction of benzene in the feed ?0?not enter ?0.40? but the cell address of this variable on the spreadsheet. In this way variances like the benzene mole fraction in the feed and the temperature and pressure of the evaporator may be changed and the new values of the calculated variables will be instantly determined. When the spreadsheet is complete use it to determine the bubble-point and dew-point temperatures of a 40 mole% benzene-.60 mole% toluene mixture at P = 800 nm H. Print out the spreadsheets for T = 100?C and for the bubble-point and dew-point temperatures.
(c) Write the code for a subprogram called FLASH to simulate the evaporator. The input variables should be the attributes of the feed stream (benzene and toluene flow rates and TF). T. P. and the physical properties of the feed stream species (Antoine constants, heat capacity formula coefficients for liquid and vapor, and heats of vaporization). The output variables are the attributes of the vapor and liquid product streams and Q. Write and run a calling program that demes me values of the feed stream variables and other input variables (use the test case values) calls the subprogram, and prints out the output variables. The physical properties may be passed to the subprogram either as arguments or through a COMMON or GLOBAL block.
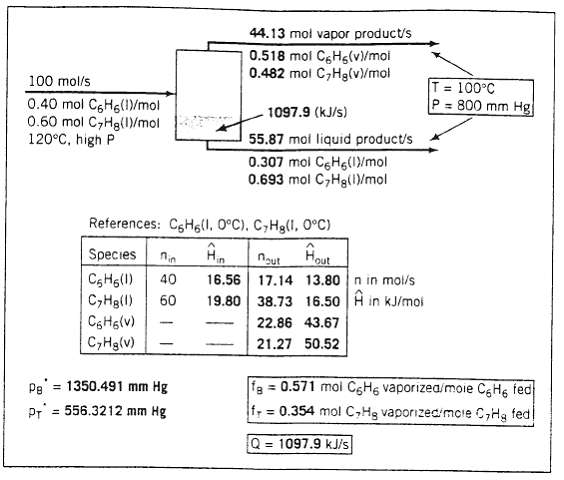
100 mol/s 0.40 mol CHg(1)/mol 0.60 mol C7Hg(1)/mol 120C, high P 44.13 mol vapor product/s 0.518 mol CHg(v)/mol 0.482 mol CHg(v)/mol References: CH(1, 0C). CyHg(1, 0C) Pe = 1350.491 mm Hg PT 556.3212 mm Hg 1097.9 (kJ/s) 55.87 mol liquid product/s 0.307 mol C6H(1)/mol 0.693 mol CHg(1)/mol Hout Species nin nout CH6(1) 40 16.56 17.14 13.80 n in mol/s CyHg(1) 60 19.80 38.73 16.50 in kJ/mol 22.86 43.67 C6H6(v) 21.27 50.52 CyHg(v) (+9 T = 100C P= 800 mm Hg fa=0.571 mol C6H6 vaporized/moie C6H6 fed f = 0.354 mol C,Hg vaporized/more C,Hg fedl Q = 1097.9 kJ/s
Step by Step Solution
3.42 Rating (165 Votes )
There are 3 Steps involved in it
a Let Bz benzene T1 toluene 106892721211033 220790 1350491 5563212 Antoine equations P 1068927212110... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (582).docx
120 KBs Word File


