Question: Here below is shown the Ellingham diagrams for some selected d-block elements. 250 Cuo Co--Co FeO C-CO SO CCO CaO 500 1000 1500 2000
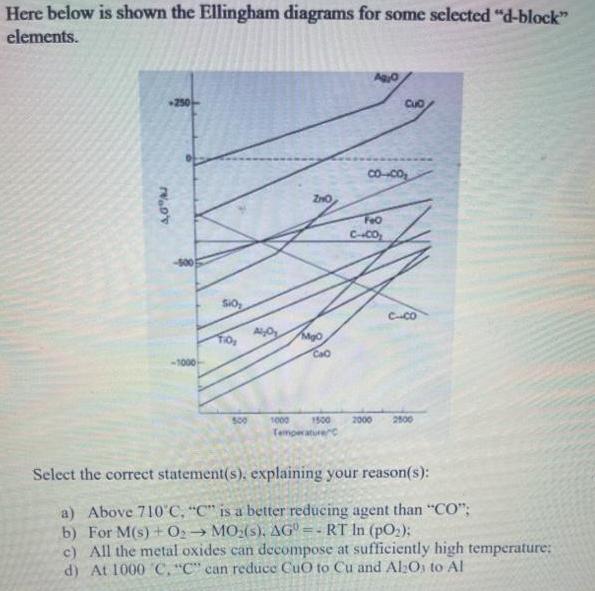
Here below is shown the Ellingham diagrams for some selected "d-block" elements. 250 Cuo Co--Co FeO C-CO SO CCO CaO 500 1000 1500 2000 2800 Temperaturec Select the correct statement(s). explaining your reason(s): a) Above 710 C, "C" is a better reducing agent than "CO"; b) For M(s)+ 02 MO:(s), AG =- RT In (pO2); c) All the metal oxides can decompose at sufficiently high temperature: d) At 1000 C, "C" can reduce CuO to Cu and Al:Os to Al
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


