Question: (b) Index the entire predicted spectrum (without assignments). While you could give more detail for the overlapping aromatic peaks, just group these into a single
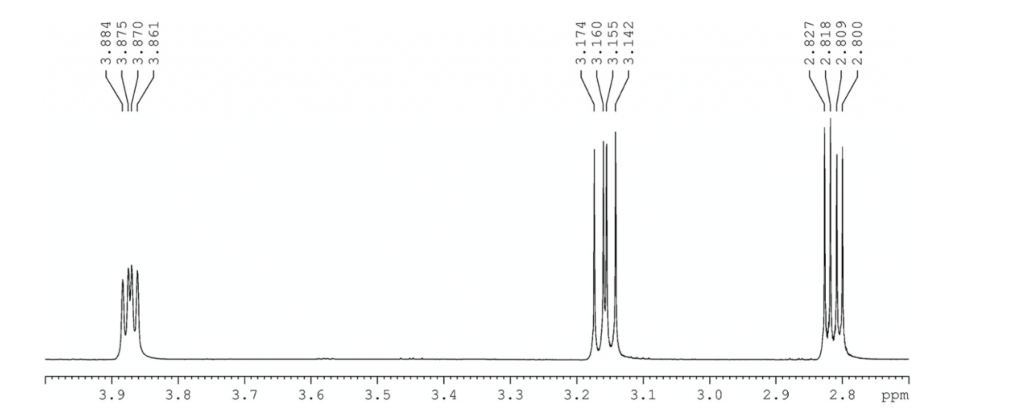
(b) Index the entire predicted spectrum (without assignments). While you could give more detail for the overlapping aromatic peaks, just group these into a single multiplet.
(c) Compare your predicted index with the experimental index below, noting where the agreement is good and not so good. Did the prediction do better on chemical shifts or coupling constants?
1H NMR (300 MHz, CDCl3) 7.28-7.40 (m, 5H), 3.87 (dd, J = 4.2, 2.7 Hz, 1H), 3.16 (dd, J = 5.6, 4.1 Hz, 1H), 2.81 (dd, J = 5.4, 2.7 Hz, 1H).
(d) The expanded spectrum in (a) makes it clear that the three H's on the 3-membered ring of styrene oxide are all different. Explain why the two H's of the CH2 are different from each other (why they have different chemical shift values, why they are in different environments).
Please answer all questions.
O 00 OO 00 00 00 00 00 00 00 00 . 174 NNNN 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 ppm O 00 OO 00 00 00 00 00 00 00 00 . 174 NNNN 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 3.1 3.0 2.9 2.8 ppm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


