Question: B. Silver - Copper Cu(s), CuCl2 (1.0 M) || Ag(s), AgNO3 (1.0 M) 1. Draw the schematic of the electrochemical cell that you created including
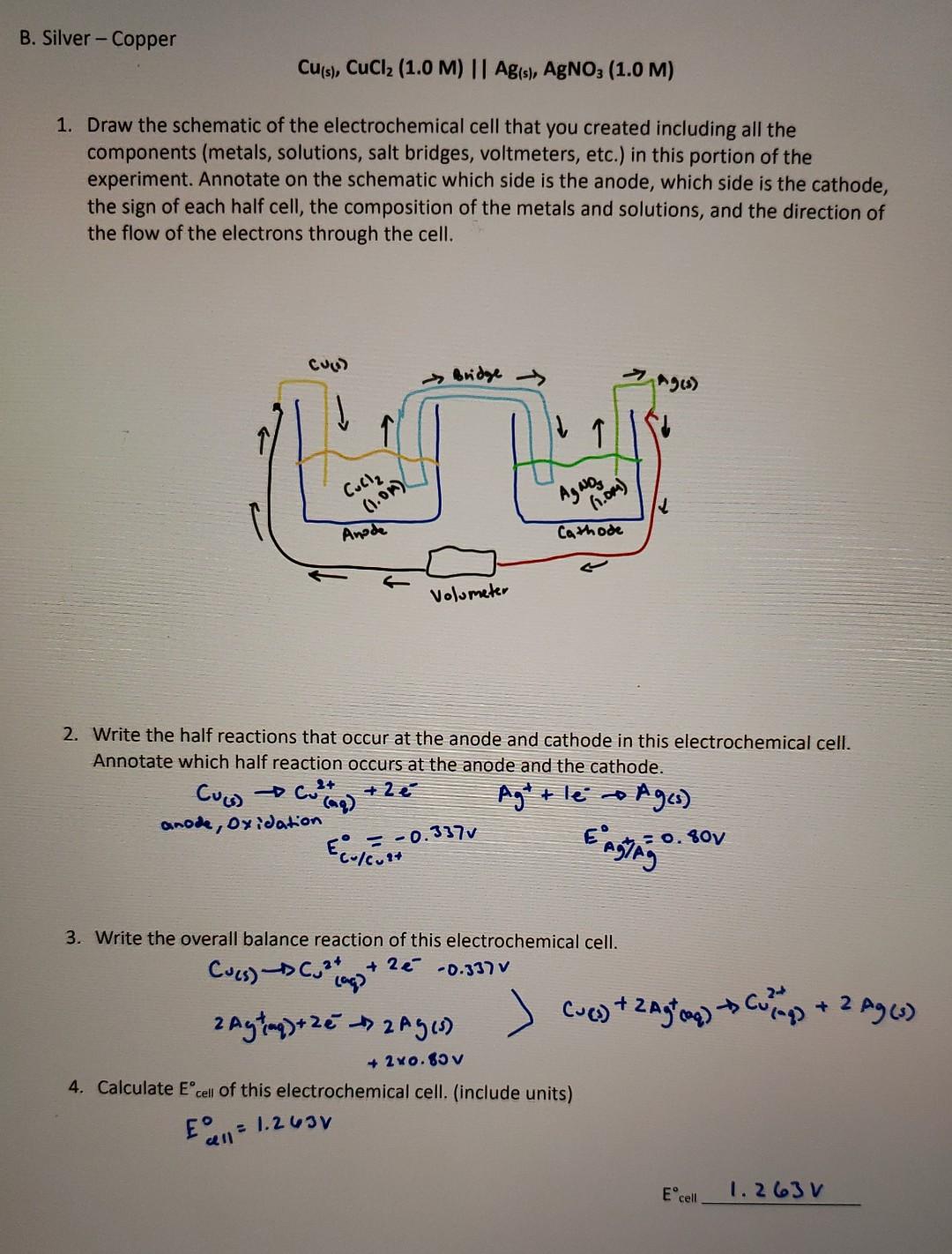
B. Silver - Copper Cu(s), CuCl2 (1.0 M) || Ag(s), AgNO3 (1.0 M) 1. Draw the schematic of the electrochemical cell that you created including all the components (metals, solutions, salt bridges, voltmeters, etc.) in this portion of the experiment. Annotate on the schematic which side is the anode, which side is the cathode, the sign of each half cell, the composition of the metals and solutions, and the direction of the flow of the electrons through the cell. CUAD Bridge Agen Culle (1.01) AgNO3 6.0m) Anode Cathode Volumeter 2. Write the half reactions that occur at the anode and cathode in this electrochemical cell. Annotate which half reaction occurs at the anode and the cathode. + 2 Cus). 2+ collag anode, oxidation Agtt le a Ages) Engang -0.337v 0.807 Ecolevat 3. Write the overall balance reaction of this electrochemical cell. Cucs).** +2 -0.337 v lag? Cuevy + zagog) to cumps + 2 Agw 2 Aytinq) + 2e Ages) + 2x0.85v 4. Calculate Ecell of this electrochemical cell. (include units) EEN = 1.263V Ecell 1.263 V 5. Calculate the reaction quotient (Q) of this reaction. Q 6. Calculate the expected Ecell for this reaction. Ecell (expected). 7. Record the value of Ecell measured for this reaction Ecell O.471 v 8. Calculate the percent error between the measured and calculated Ecell for this reaction. What are some of the possible ces of this error? Is the error value reaso % error
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


