Question: B. The elementary irreversible organic liquid phase reaction 2 A+BC +2D 3 is carried out in a flow reactor with a constant volumetric flowrate of
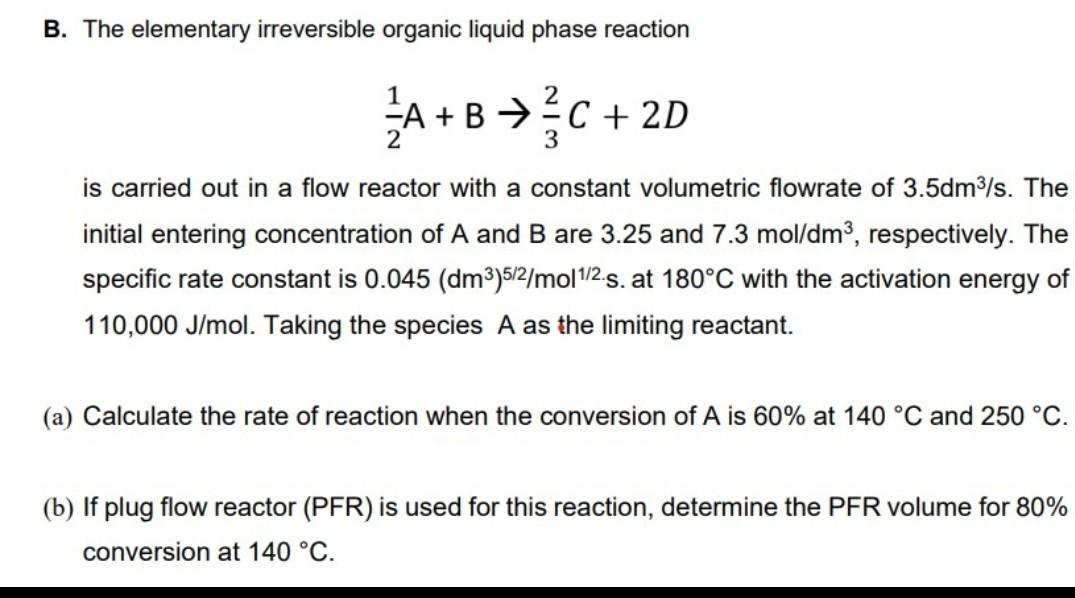
B. The elementary irreversible organic liquid phase reaction 2 A+BC +2D 3 is carried out in a flow reactor with a constant volumetric flowrate of 3.5dm3/s. The initial entering concentration of A and B are 3.25 and 7.3 mol/dm3, respectively. The specific rate constant is 0.045 (dm3)512/mol 1/2.s. at 180C with the activation energy of 110,000 J/mol. Taking the species A as the limiting reactant. (a) Calculate the rate of reaction when the conversion of A is 60% at 140 C and 250 C. (b) If plug flow reactor (PFR) is used for this reaction, determine the PFR volume for 80% conversion at 140 C
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


