Question: s The elementary irreversible liquid - phase reaction A + B C is carried out in a CSTR , and a conversion of 7 0
s The elementary irreversible liquidphase reaction A B C is carried out in a CSTR and a conversion of Problem
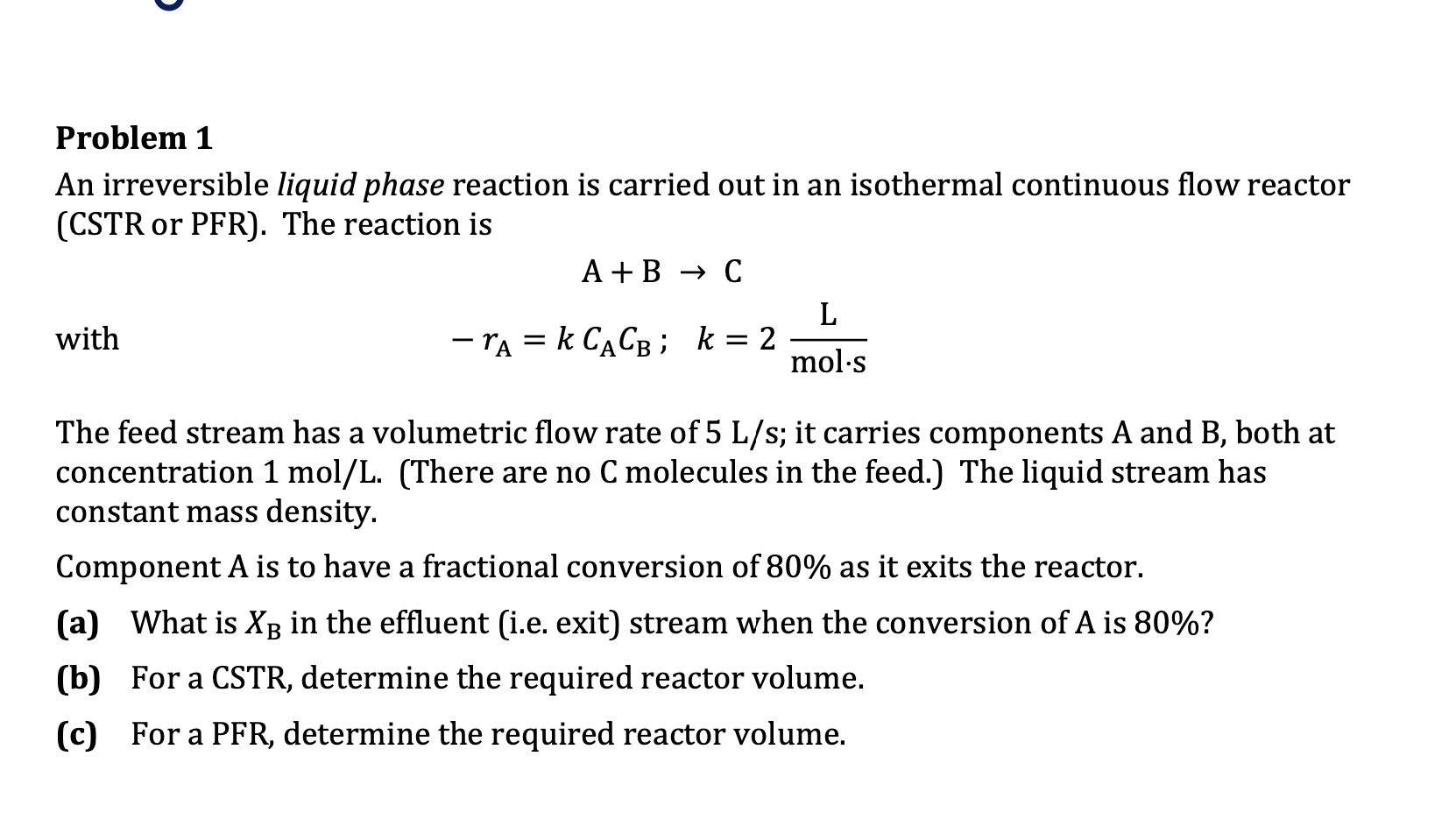
An irreversible liquid phase reaction is carried out in an isothermal continuous flow reactor
CSTR or PFR The reaction is
with
;
The feed stream has a volumetric flow rate of ; it carries components A and both at
concentration There are no molecules in the feed. The liquid stream has
constant mass density.
Component is to have a fractional conversion of as it exits the reactor.
a What is in the effluent ie exit stream when the conversion of is
b For a CSTR determine the required reactor volume.
c For a PFR determine the required reactor volume.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


