Question: b) The following data in Table 1 are measured for the reaction of nitric oxide with hydrogen: 2NO(g)+2H2(g)N2(g)+2H2O(g) Table 1: Rate for the reaction of
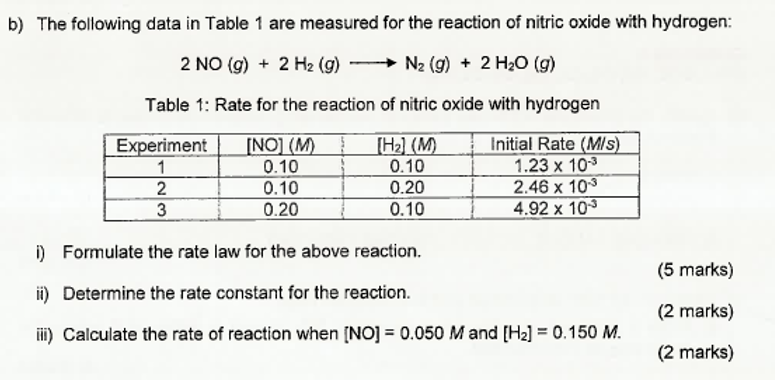
b) The following data in Table 1 are measured for the reaction of nitric oxide with hydrogen: 2NO(g)+2H2(g)N2(g)+2H2O(g) Table 1: Rate for the reaction of nitric oxide with hydrogen i) Formulate the rate law for the above reaction. (5 marks) ii) Determine the rate constant for the reaction. (2 marks) iii) Calculate the rate of reaction when [NO]=0.050M and [H2]=0.150M. (2 marks)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


