Question: (b) The following Table 2 shows kinetic study values obtained from elementary step number 2. Table 2 T(K) 273 298 308 318 k (cm mol
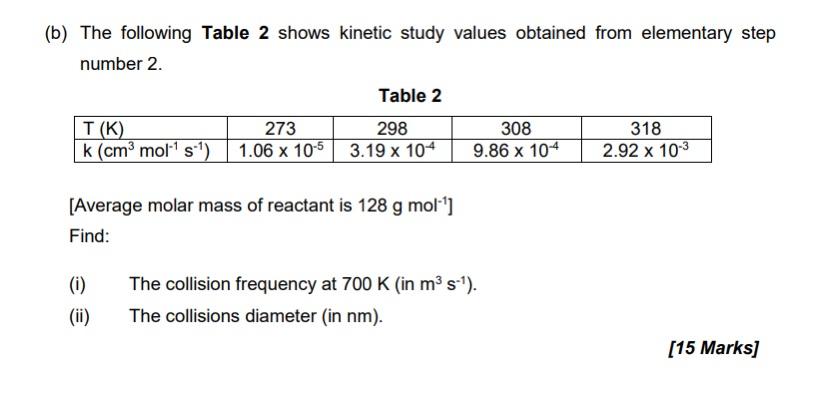
(b) The following Table 2 shows kinetic study values obtained from elementary step number 2. Table 2 T(K) 273 298 308 318 k (cm mol st) 1.06 x 10-5 3.19 x 104 9.86 x 10-4 2.92 x 10-3 [Average molar mass of reactant is 128 g mol'] Find: 0) (i) (ii) The collision frequency at 700 K (in ms). The collisions diameter (in nm). [15 Marks] (b) The following Table 2 shows kinetic study values obtained from elementary step number 2. Table 2 T(K) 273 298 308 318 k (cm mol st) 1.06 x 10-5 3.19 x 104 9.86 x 10-4 2.92 x 10-3 [Average molar mass of reactant is 128 g mol'] Find: 0) (i) (ii) The collision frequency at 700 K (in ms). The collisions diameter (in nm). [15 Marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


