Question: (b) The following Table 2 shows kinetic study values obtained from elementary step number 2. + CH3CHO HI + CH.CO Table 2 T(K) 273 298
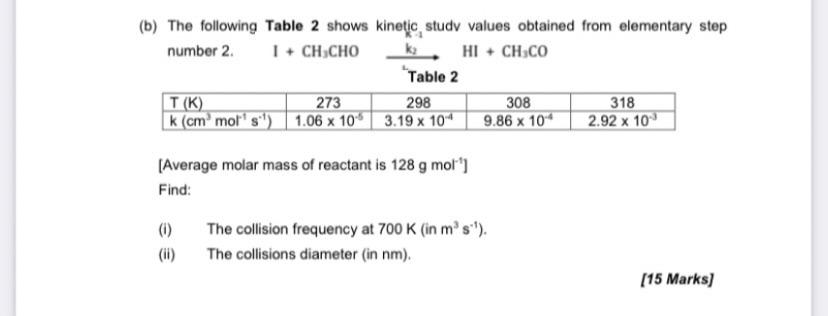
(b) The following Table 2 shows kinetic study values obtained from elementary step number 2. + CH3CHO HI + CH.CO Table 2 T(K) 273 298 308 318 k (cm' molt' si) 1.06 x 10" 3.19 x 10 9.86 x 10 2.92 x 10 (Average molar mass of reactant is 128 g mol") Find: (6) The collision frequency at 700 K (in ms'). The collisions diameter (in nm). [15 Marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


