Question: b) With reference to the data in Figure Q5: to identify whether the mixture of methanol and chloroform is ideal or non-ideal. With reference to
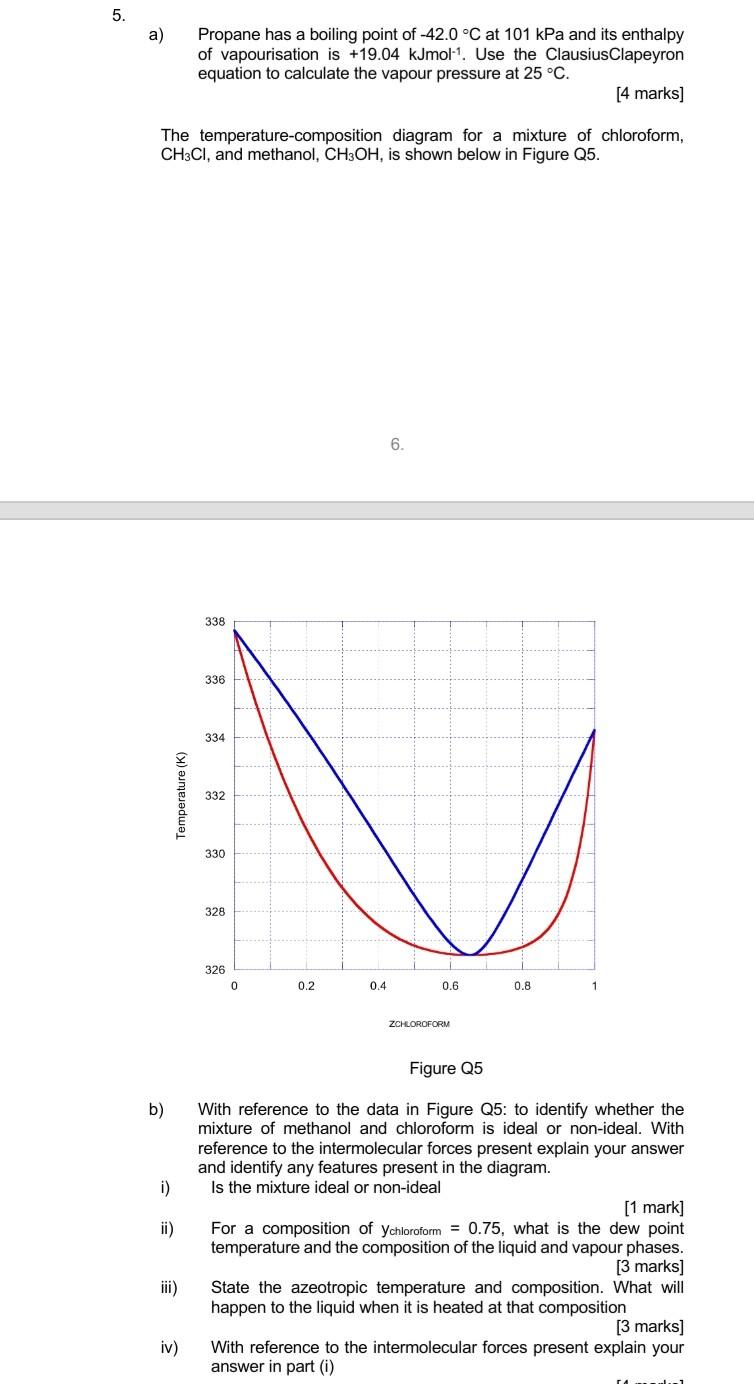
b) With reference to the data in Figure Q5: to identify whether the mixture of methanol and chloroform is ideal or non-ideal. With reference to the intermolecular forces present explain your answer and identify any features present in the diagram. i) Is the mixture ideal or non-ideal [1 mark] ii) For a composition of ychloroform = 0.75, what is the dew point temperature and the composition of the liquid and vapour phases. [3 marks] iii) State the azeotropic temperature and composition. What will happen to the liquid when it is heated at that composition [3 marks] iv) With reference to the intermolecular forces present explain your answer in part (i)
5. a) Propane has a boiling point of -42.0 C at 101 kPa and its enthalpy of vapourisation is +19.04 kJmol-1. Use the Clausius Clapeyron equation to calculate the vapour pressure at 25C. [4 marks] The temperature-composition diagram for a mixture of chloroform, CH3CI, and methanol, CH3OH, is shown below in Figure Q5. 6. 338 336 334 Temperature (K) 332 330 328 1 326 0 0.2 0.4 0.6 0.8 1 ZCHLOROFORM Figure Q5 b) i) ii) With reference to the data in Figure Q5: to identify whether the mixture of methanol and chloroform is ideal or non-ideal. With reference to the intermolecular forces present explain your answer and identify any features present in the diagram. Is the mixture ideal or non-ideal [1 mark] For a composition of ychloroform = 0.75, what is the dew point temperature and the composition of the liquid and vapour phases. [3 marks) State the azeotropic temperature and composition. What will happen to the liquid when it is heated at that composition [3 marks] With reference to the intermolecular forces present explain your answer in part (1) iii) iv) ra_1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


