Question: Balance the following reaction using either the half reaction method or the oxidation number method in a basic solution. Zn(s)+NO3(aq)ZnO22(aq)+NH3(g) A **Please show all your
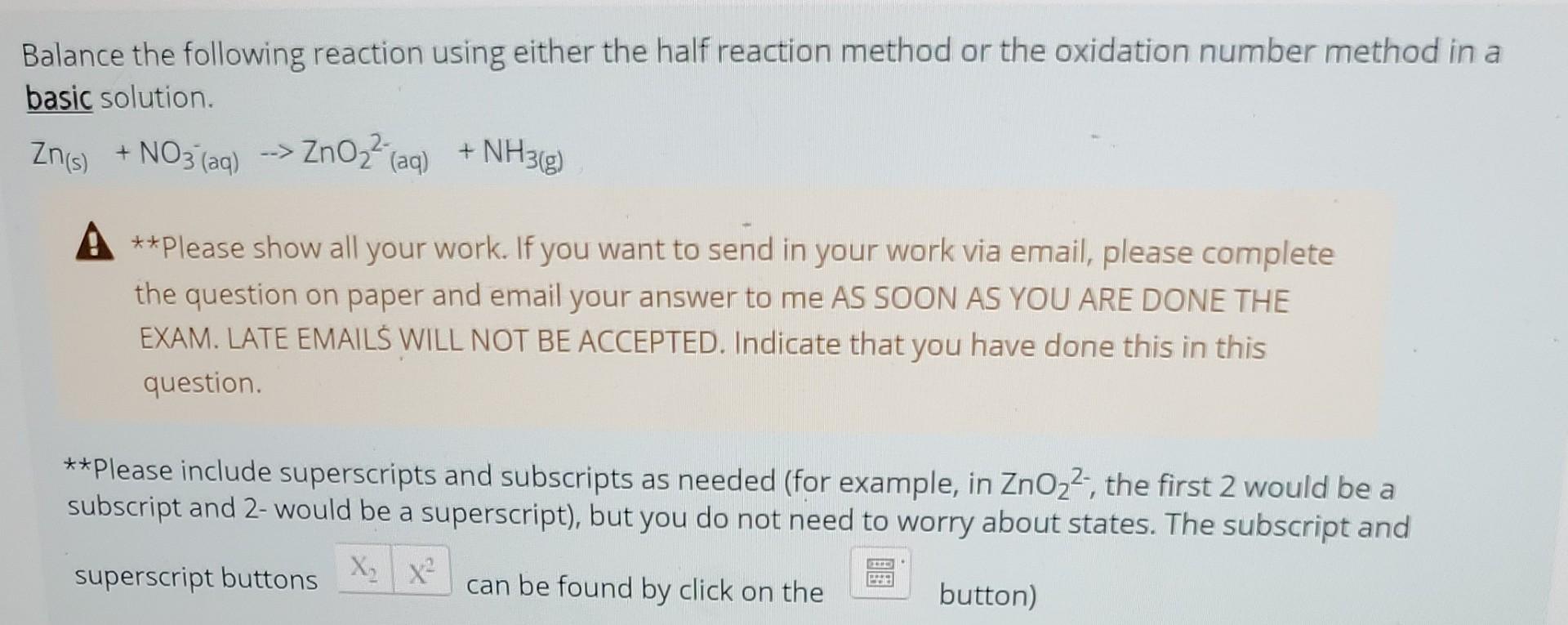
Balance the following reaction using either the half reaction method or the oxidation number method in a basic solution. Zn(s)+NO3(aq)ZnO22(aq)+NH3(g) A **Please show all your work. If you want to send in your work via email, please complete the question on paper and email your answer to me AS SOON AS YOU ARE DONE THE EXAM. LATE EMAILS WILL NOT BE ACCEPTED. Indicate that you have done this in this question. **please include superscripts and subscripts as needed (for example, in ZnO22, the first 2 would be a subscript and 2-would be a superscript), but you do not need to worry about states. The subscript and superscript buttons can be found by click on the button)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


