Question: Use the References to access important values if needed for this question. A helium-filled weather balloon has a volume of 695L at 14.9C and 755mmHg.
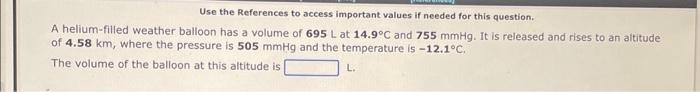
Use the References to access important values if needed for this question. A helium-filled weather balloon has a volume of 695L at 14.9C and 755mmHg. It is released and rises to an altitude The volume of the balloon at this altitude is L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


