Question: Based on the buffer you have identified in the previous step, the molar mass of the salt used to prepare this buffer is: This
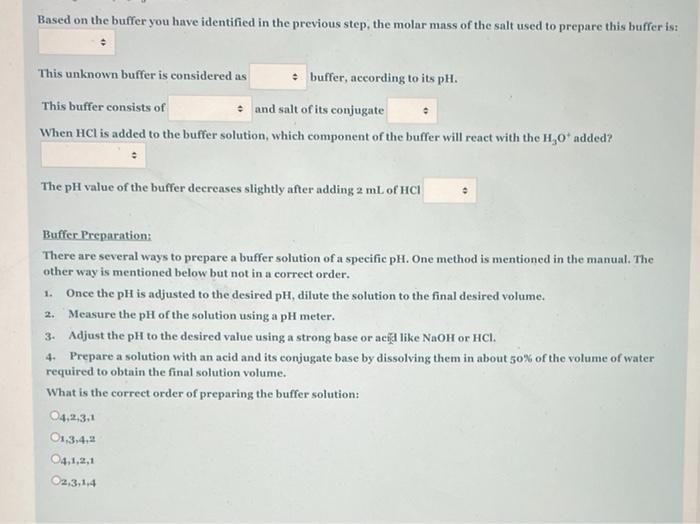
Based on the buffer you have identified in the previous step, the molar mass of the salt used to prepare this buffer is: This unknown buffer is considered as * buffer, according to its pH. This buffer consists of * and salt of its conjugate When HCl is added to the buffer solution, which component of the buffer will react with the H,0 added? The pH value of the buffer decreases slightly after adding 2 ml. of HCI Buffer Preparation: There are several ways to prepare a buffer solution of a specific pH. One method is mentioned in the manual. The other way is mentioned below but not in a correct order. 1. Once the pH is adjusted to the desired pH, dilute the solution to the final desired volume. 2. Measure the pH of the solution using a pH meter. 3. Adjust the pH to the desired value using a strong base or acid like NaOH or HCI. 4. Prepare a solution with an acid and its conjugate base by dissolving them in about s0% of the volume of water required to obtain the final solution volume. What is the correct order of preparing the buffer solution: 04,2,3.1 O13.4.2 O4.1,2,1 O2,3,14
Step by Step Solution
3.30 Rating (150 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


