Question: Preparation of buffer solution and identification of unknown buffer solution Part I: Determination of K, of the buffer solution Solution pH Initial After adding
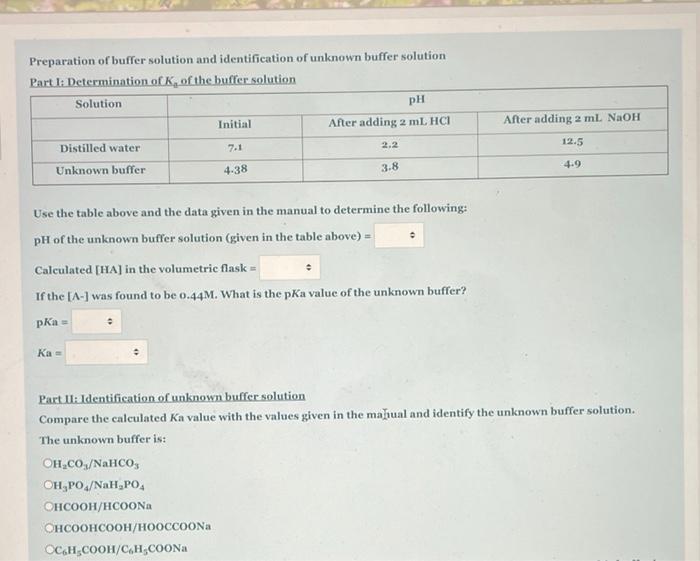
Preparation of buffer solution and identification of unknown buffer solution Part I: Determination of K, of the buffer solution Solution pH Initial After adding 2 mL HCI After adding 2 ml NaOH Distilled water 7-1 12.5 2.2 Unknown buffer 4-38 3-8 4.9 Use the table above and the data given in the manual to determine the following: pH of the unknown buffer solution (given in the table above) = Calculated [HA] in the volumetric flask = If the [A-] was found to be o.44M. What is the pka value of the unknown buffer? pKa = Ka = Part II: Identification of unknown buffer solution Compare the calculated Ka value with the values given in the mahual and identify the unknown buffer solution. The unknown buffer is: OH,CO./NaHCO, OH,PO,/NaH, PO, /a /Na OC,H;COOH/C,H;COONA
Step by Step Solution
3.44 Rating (170 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


