Question: based on the data from the following experiment answer the questions experiment Iodimetry Titration: Redox titration of ascorbic acid with Iodine Part B: Procedure Analysis
based on the data from the following experiment answer the questions
experiment Iodimetry Titration: Redox titration of ascorbic acid with Iodine
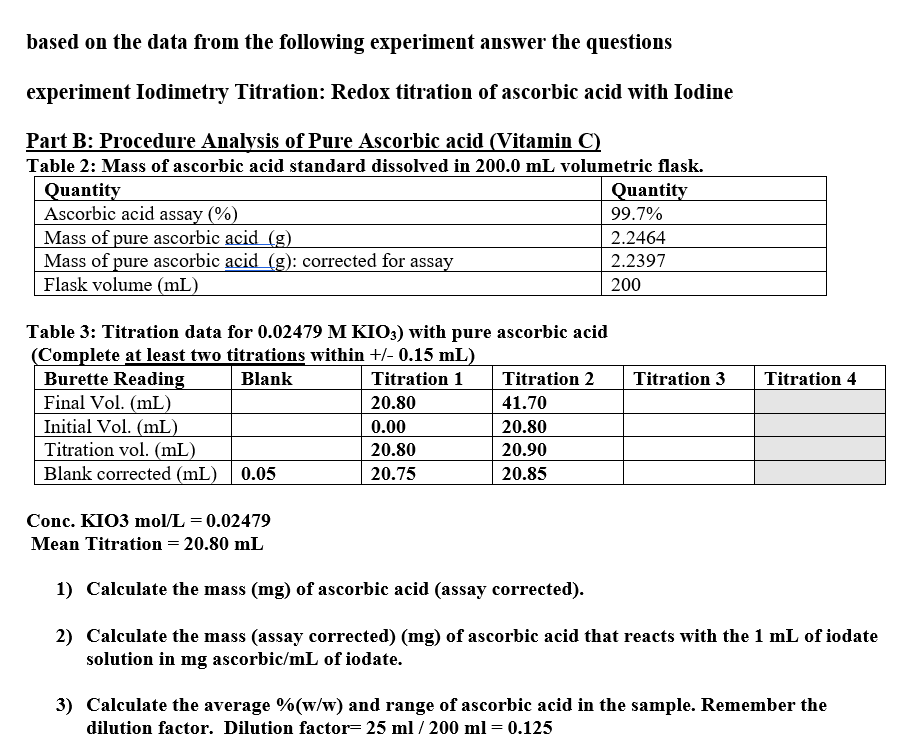
Part B: Procedure Analysis of Pure Ascorbic acid Vitamin C
Table : Mass of ascorbic acid standard dissolved in volumetric flask.
Table : Titration data for with pure ascorbic acid
Complete at least two titrations within
Conc. KIO
Mean Titration
Calculate the mass mg of ascorbic acid assay corrected
Calculate the mass assay correctedmg of ascorbic acid that reacts with the of iodate
solution in ascorbicmL of iodate.
Calculate the average and range of ascorbic acid in the sample. Remember the
dilution factor. Dilution factor
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


