Question: based on the data from the following experiment answer the questions experiment: Iodimetry titration: redox titration of vitamin C with iodine Part D: Analysis of
based on the data from the following experiment answer the questions
experiment: Iodimetry titration: redox titration of vitamin with iodine
Part D: Analysis of Vitamin C in Fruit Juices
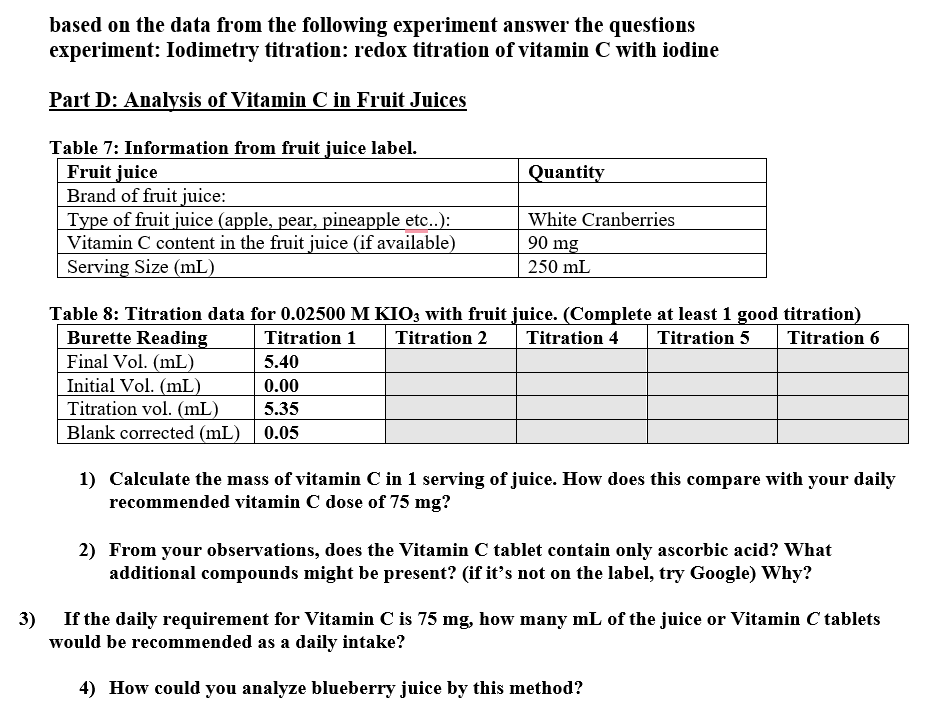
Table : Information from fruit juice label.
Table : Titration data for with fruit juice. Complete at least good titration
Calculate the mass of vitamin in serving of juice. How does this compare with your daily
recommended vitamin dose of
From your observations, does the Vitamin tablet contain only ascorbic acid? What
additional compounds might be present? if it's not on the label, try Google Why?
If the daily requirement for Vitamin is how many of the juice or Vitamin tablets
would be recommended as a daily intake?
How could you analyze blueberry juice by this method?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


