Question: based on the density refer table 2 common solvent and identify your unknown liquid and the density and identification Copper Unknown Solid: Be sure to

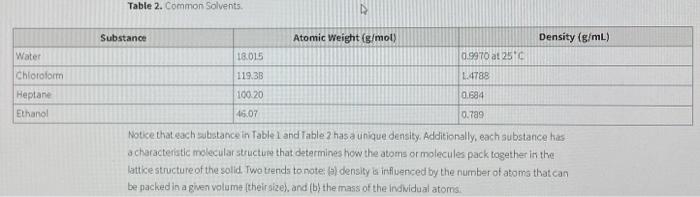
Copper Unknown Solid: Be sure to include the density and Identification of your unknown in your conclusion. 7. Unknown Ilquid: B Measurement Trial 1 Trial 2 Mass of graduated cylinder (9) 37.3709 Zr 21.380 Volume of liquid (ml.) 8.7 MORO 10.7m ! Mass of graduated cylinder and liquid (9) 32.347921.42119 Mass of liquid (9) 4.9679 0.04331 Density of liquid (g/mL) Average density of unknown liquid (g/mL) 21.63184all 8. Calculate density of Unknown liquid. Show calcuation below. Use correct significant figures. Show Formula: D-MW. Plug in data with units. Block in answer with units. 4 3.2 lagini 143.212g/ml .58168lgimi - - 21.86 4855 g/ml 13.2.139.10kasoyles. 51(819 m) (4.9679) (8.7mi) 10.04839 ) (10.741) 581681 simi Based on the density, refer to Table 2: Common Solvents and identify your Unknown liquid. known Liquid: sure to include the density and identification of unknown in your Conclusion. Table 2. Common Solvents. N Water Chloroform Heplane Ethanol Substance Atomic Weight (g/mol) Density (g/mL) 18.015 0.9970 at 256 119.30 L. 4789 100.20 0.594 46.07 0.789 Note that each substance in Table and Table 7 has a unique density. Additionally, each substance has a characteristic molecular structure that determines how the atoms or molecules pack together in the lattice structure of the solid. Two trends to note: a) density influenced by the number of atoms that can be packed in a given volume (their size) and (b) the mass of the individual atoms
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


