Question: Based on the following graph, which process would release the most energy on freezing: 100.0 g of water or 100.0 g of diethyl ether and
Based on the following graph, which process would release the most energy on freezing: 100.0 g of water or 100.0 g of diethyl ether and why? Explain HOW you would go about solving the problem AND identify the equations you would use. Bonus: Provide actual calculations demonstrating which substance would release more energy. 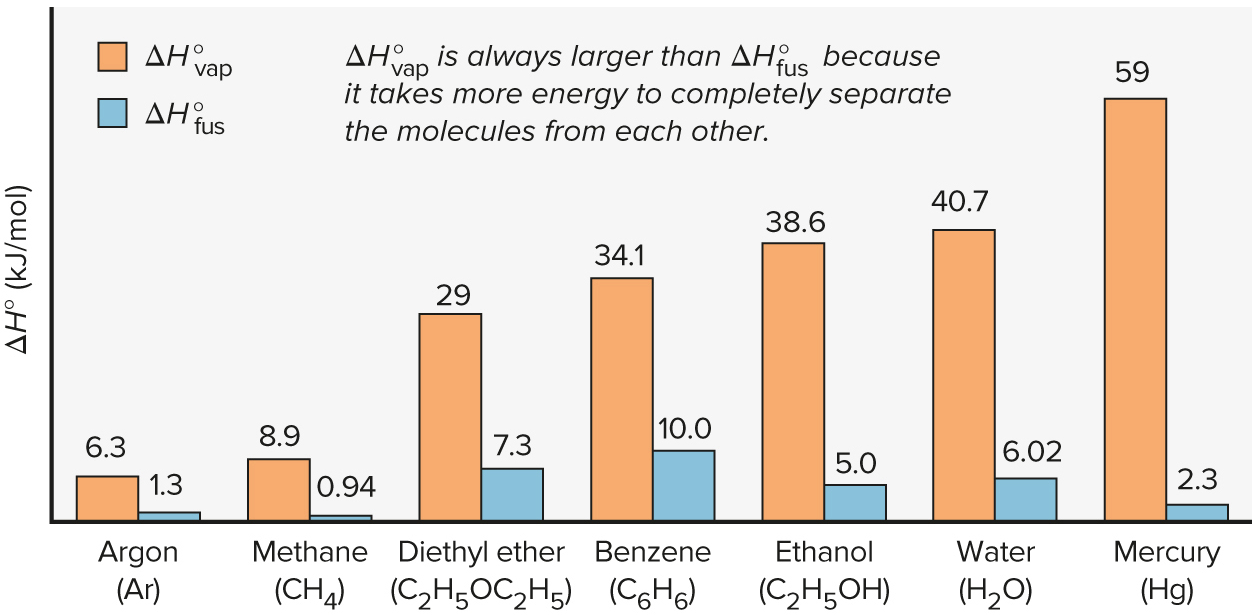
. 10 vap 59 AHvap is always larger than AHfus because it takes more energy to completely separate the molecules from each other. . o fus 40.7 38.6 34.1 AH (kJ/mol) 29 6.3 8.9 10.0 7.3 5.0 6.02 1.3 0.94 2.3 Argon (Ar) Methane Diethyl ether Benzene (CH4) (C2H5OC2H5) (C6H6) Ethanol (C2H5OH) Water (H20) Mercury (Hg)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


