Question: Based on what you learned in the simulation, consider these properties for a mystery element: Roanestatium with only three isotopes: Rs-30 with a mass of

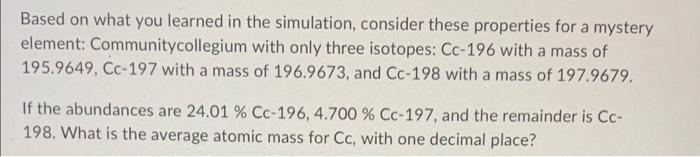
Based on what you learned in the simulation, consider these properties for a mystery element: Roanestatium with only three isotopes: Rs-30 with a mass of 29.97376, Rs32 with a mass of 31.97207, and Rs-34 with a mass of 33.96786. If the abundances are 1.240% Rs-30, 91.30% Rs-32, and the remainder is Rs-34. What is the average atomic mass for Rs, with 2 decimal places? Based on what you learned in the simulation, consider these properties for a mystery element: Communitycollegium with only three isotopes: Cc-196 with a mass of 195.9649, Cc 197 with a mass of 196.9673, and Cc-198 with a mass of 197.9679. If the abundances are 24.01%Cc196,4.700%Cc197, and the remainder is Cc198. What is the average atomic mass for Cc, with one decimal place
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


