Question: Based on your answer above, how is the unit cell of TlCl(s)likely to be structured? even that the density of TICI(s) is 7.00 g/cm and
Based on your answer above, how is the unit cell of TlCl(s)likely to be structured?
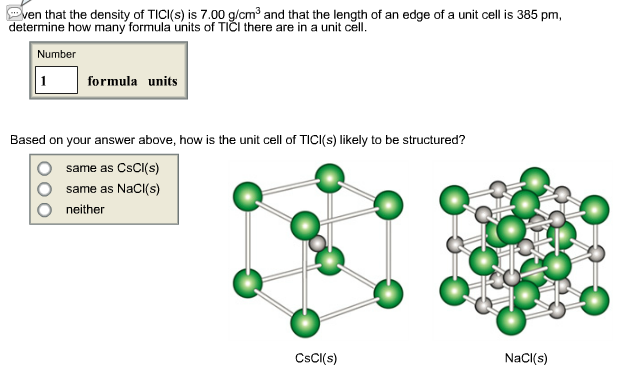
even that the density of TICI(s) is 7.00 g/cm and that the length of an edge of a unit cell is 385 pm, determine how many formula units of TII there are in a unit cell. Number 1 formula units Based on your answer above, how is the unit cell of TICI(s) likely to be structured? same as CsCl(s) same as NaCl(s) neither CsCl(s) NaCl(s)
Step by Step Solution
3.50 Rating (153 Votes )
There are 3 Steps involved in it
Answer Given data Density of TICI s d 700 gcm Edge of unit cell ... View full answer

Get step-by-step solutions from verified subject matter experts


