Question: Item 4 An aqueous KNO3 solution is made using 74.1 g of KNO3 diluted to a total solution volume of 2.06 L. Part A
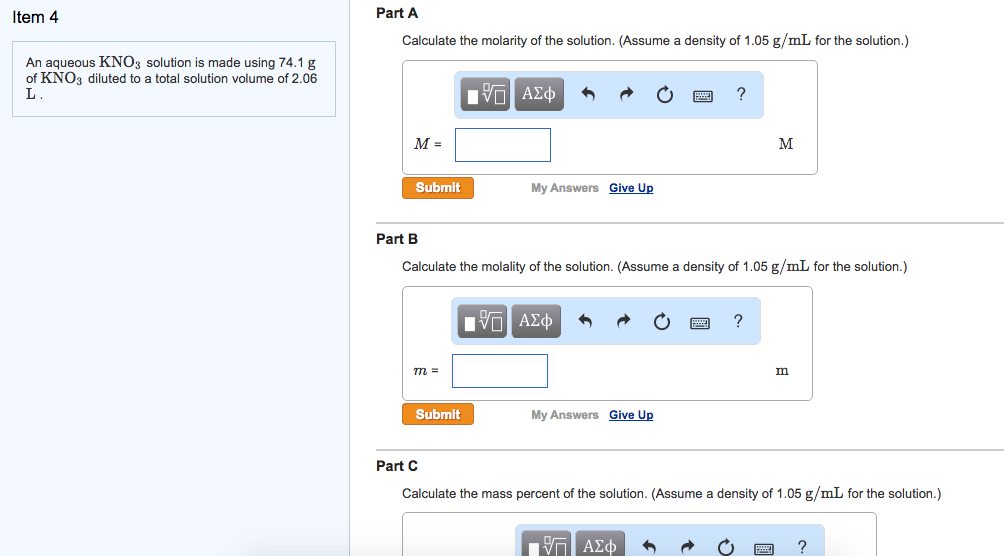
Item 4 An aqueous KNO3 solution is made using 74.1 g of KNO3 diluted to a total solution volume of 2.06 L. Part A Calculate the molarity of the solution. (Assume a density of 1.05 g/mL for the solution.) M = Submit m = IVE Submit My Answers Give Up Part B Calculate the molality of the solution. (Assume a density of 1.05 g/mL for the solution.) VE My Answers Give Up ? { M ? m Part C Calculate the mass percent of the solution. (Assume a density of 1.05 g/mL for the solution.) ?
Step by Step Solution
3.47 Rating (150 Votes )
There are 3 Steps involved in it
PartA Molarity M Part B massg density x volume 105 gmL x ... View full answer

Get step-by-step solutions from verified subject matter experts


