Question: be nice and answer me properly now it's the second time I ask and it costs me money. Can you draw or indicate on a


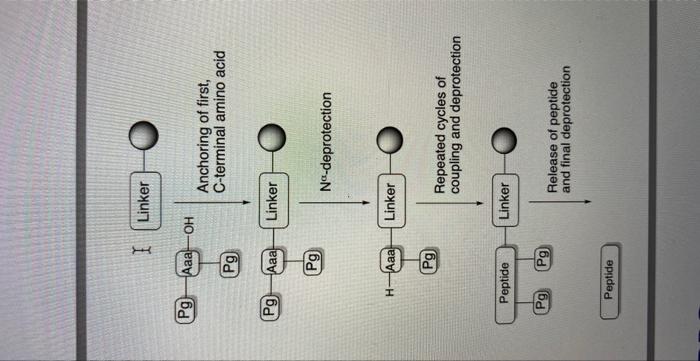
The synthesis of the peptide H-Leu-Ala-HisTyr-Asn-Lys-NH2 using Fmoc-based solidphase synthesis requires the following steps: Use cross-linked polystyrene resin as the solid support and 4-chloromethylphenoxybenzyl as the linker. Protect the N-terminal with Fmoc and the side chains of His, Tyr, and Asn with DCB, CT, and Trt, respectively. Use N,N'-dicyclohexylcarbodiimide (DCC) as the coupling reagent. Use Leu, Ala, His, Tyr, Asn, and Lys as the amino acid building blocks. Load the resin, attach the linker, deprotect the N-terminal Fmoc group, and add the first amino acid, Leu. Repeat the process of deprotection, coupling, and washing for each subsequent amino acid in the peptide sequence. Final deprotection, cleavage from the resin, and purification to obtain the final peptide. Provide a detailed plan and explanation on how to synthesize the following peptide by using Fmoc-based solid phase synthesis: H-Leu-Ala-His-Tyr-Asn-Lys-NH2 The written report should include a description of the choice of resin, linker, protecting groups, coupling reagents, amino acid 'building blocks', etc. needed in order to conduct this synthesis. Peptide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


