Question: Be sure to answer all parts. Three 8-L flasks, fixed with pressure gauges and small valves, each contain 7g of gas at 276K. Flask A
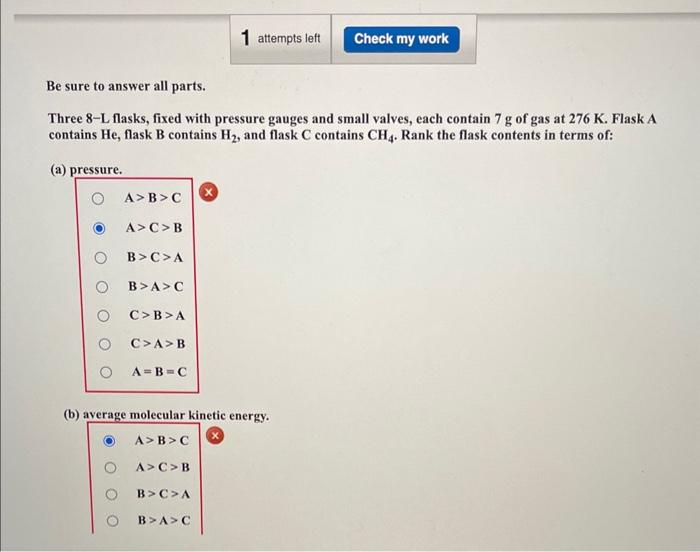
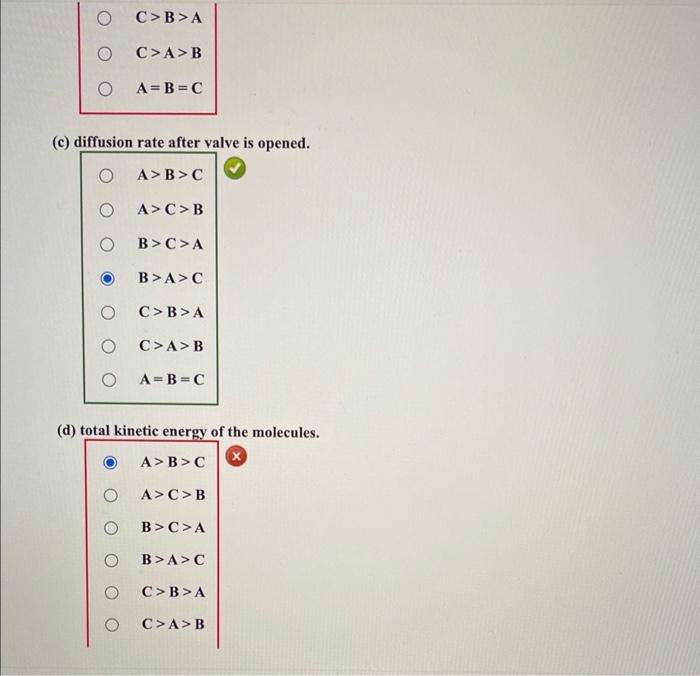
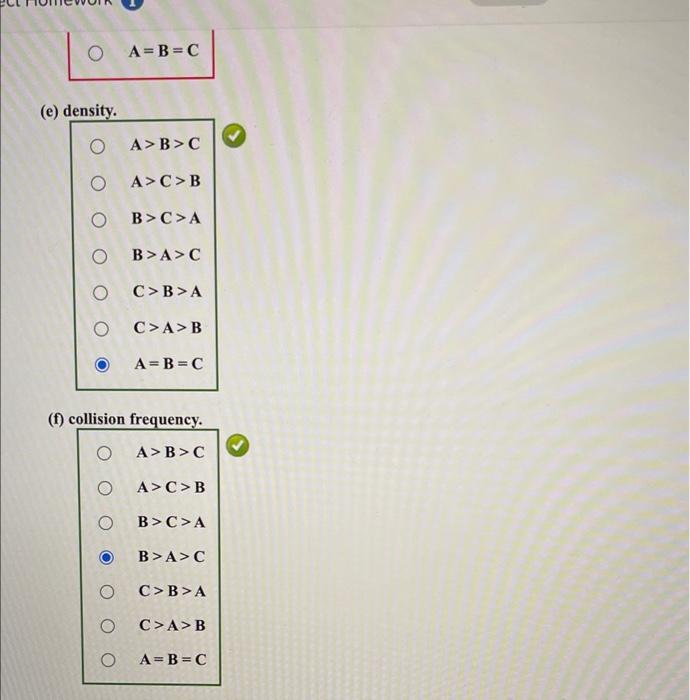
Be sure to answer all parts. Three 8-L flasks, fixed with pressure gauges and small valves, each contain 7g of gas at 276K. Flask A contains He, flask B contains H2, and flask C contains CH4. Rank the flask contents in terms of: (a) pressure. A>B>C A>C>B B>C>A B>A>C C>B>A C>A>B A=B=C (b) average molecular kinetic energy. A>B>CA>C>BB>C>AB>A>C C>B>AC>A>BA=B=C (c) diffusion rate after valve is opened. A>B>C A>C>B B>C>A B>A>C C>B>A C>A>B A=B=C (d) total kinetic energy of the molecules. A>B>C A>C>B B>C>A B>A>C C>B>A C>A>B A=B=C (e) density. A>B>CA>C>BB>C>AB>A>CC>B>AC>A>BA=B=C (f) collision frequency. A>B>CA>C>BB>C>AB>A>CC>B>AC>A>BA=B=C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


