Question: Beer's Law Lab Report!!! please help, show work!!! 4. Make a graph of the spectrum of the stock solution of cobalt (II) chloride hydrate. NO

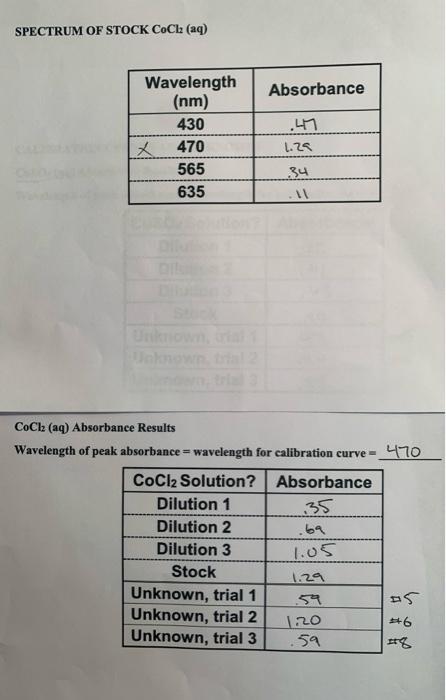
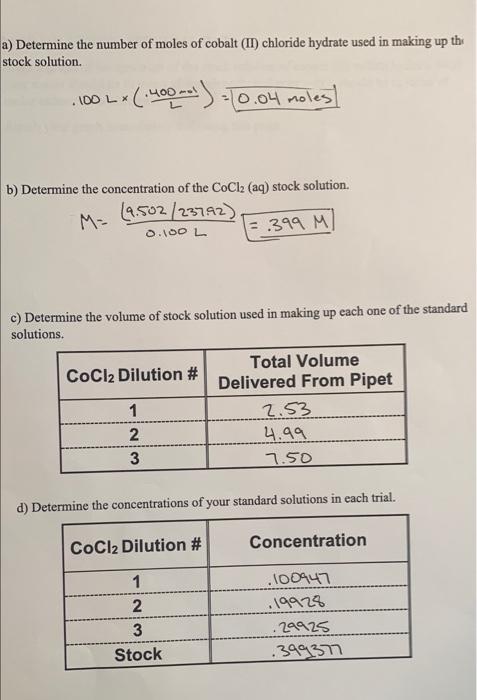
4. Make a graph of the spectrum of the stock solution of cobalt (II) chloride hydrate. NO your x-axis should be in terms of wavelength, and your y-axis should be in terms of molar extinction coefficients. Attach your graph immediately following this page. 6. Make a calibration curve for cobalt(II) chloride hydrate. Include both the best-fit-line' equation and its R-squared value. Attach your graph immediately following this page. 9. Calculate the average concentration of the CoCl2 (aq) unknown, using your data and yo calibration curve. 10. a) Determine the standard deviation for the CoCl2 (aq) unknown's concentration. SPECTRUM OF STOCK COCl (aq) Absorbance .49 Wavelength (nm) 430 X 470 565 635 L.25 134 CoCl2 (aq) Absorbance Results Wavelength of peak absorbance = wavelength for calibration curve 470 CoCl2 Solution? Absorbance Dilution 1 .35 Dilution 2 .ba Dilution 3 Stock 1.29 Unknown, trial 1 Unknown, trial 2 1:20 Unknown, trial 3 .59 It8 1.05 57 a) Determine the number of moles of cobalt(II) chloride hydrate used in making up th stock solution. . 100 LX (400)=10.04 moles] b) Determine the concentration of the CoCl2 (aq) stock solution. M= 9.502/237.92) = 399 M 0.100L c) Determine the volume of stock solution used in making up each one of the standard solutions. Total Volume CoCl2 Dilution # Delivered From Pipet 1 2.53 2 4.99 3 7.50 WIN d) Determine the concentrations of your standard solutions in each trial. CoCl2 Dilution # Concentration 1 2 3 Stock 100947 19928 29925 .39937
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


