Question: Before this laboratory, you should: - Read the lab handout until you have a clear sense of what you will be doing. - In your
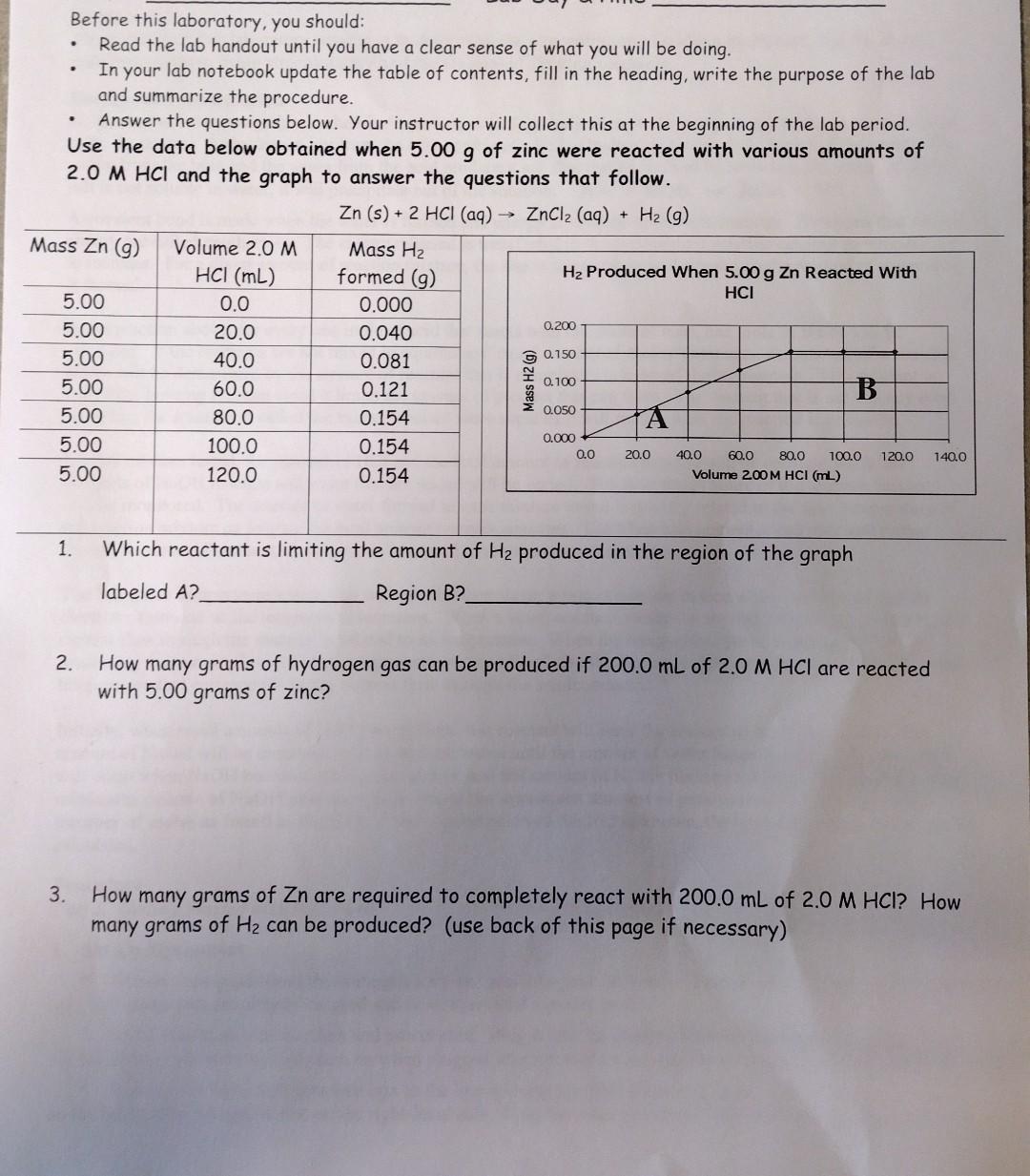
Before this laboratory, you should: - Read the lab handout until you have a clear sense of what you will be doing. - In your lab notebook update the table of contents, fill in the heading, write the purpose of the lab and summarize the procedure. - Answer the questions below. Your instructor will collect this at the beginning of the lab period. Use the data below obtained when 5.00g of zinc were reacted with various amounts of 2.0 MHCl and the graph to answer the questions that follow. Zn(s)+2HCl(aq)ZnCl2(aq)+H2(g) 1. Which reactant is limiting the amount of H2 produced in the region of the graph labeled A ? Region B? 2. How many grams of hydrogen gas can be produced if 200.0mL of 2.0MHCl are reacted with 5.00 grams of zinc? 3. How many grams of Zn are required to completely react with 200.0mL of 2.0MHCl ? How many grams of H2 can be produced? (use back of this page if necessary)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


