Question: begin{tabular}{lll} Substance & cal /gC & J/gC hline Elements & & Aluminum, Al(s) & 0.214 & 0.897 Copper, Cu(s) & 0.0920 &
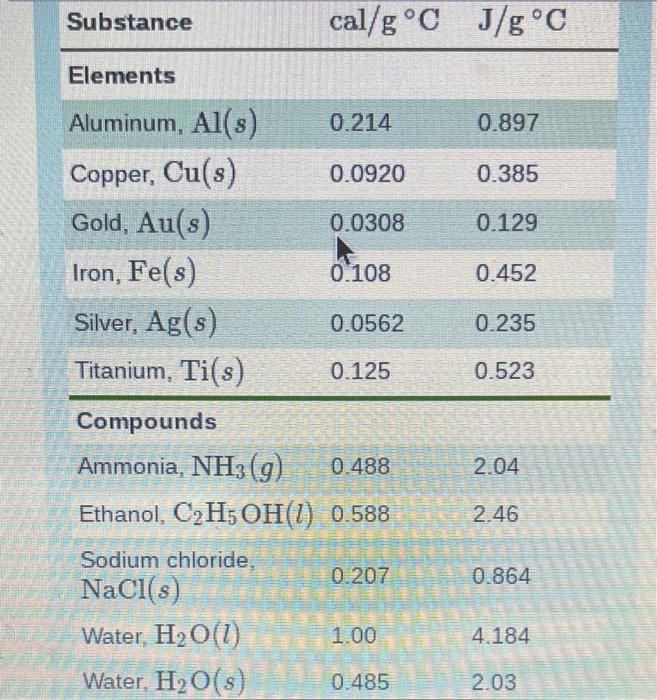

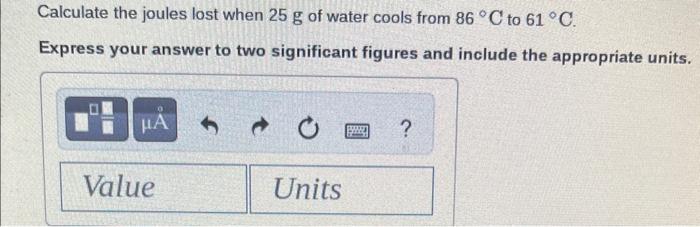
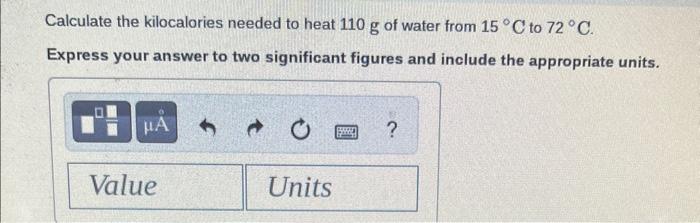
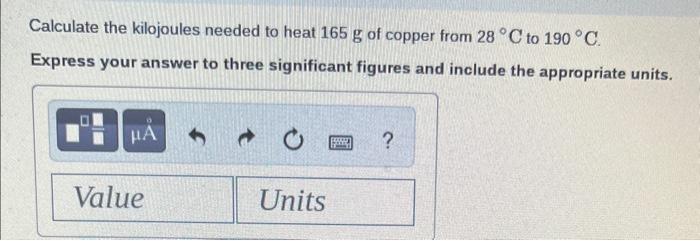
\begin{tabular}{lll} Substance & cal /gC & J/gC \\ \hline Elements & & \\ Aluminum, Al(s) & 0.214 & 0.897 \\ Copper, Cu(s) & 0.0920 & 0.385 \\ Gold, Au(s) & 0.0308 & 0.129 \\ Iron, Fe(s) & 0.108 & 0.452 \\ Silver,Ag(s) & 0.0562 & 0.235 \\ Titanium, Ti(s) & 0.125 & 0.523 \\ \hline Compounds & & \\ Ammonia, NH \\ Ethanol, C2H5OH(g) & 0.488 & 2.04 \\ Sodium chloride, & 0.588 & 2.46 \\ NaCl (s) & 0.207 & 0.864 \\ Water, H2O(l) & 1.00 & 4.184 \\ Water, H2O(s) & 0.485 & 2.03 \end{tabular} Calculate the calories needed to heat 9.8g of water from 15C to 33C. Express your answer to two significant figures and include the appropriate units. Calculate the joules lost when 25g of water cools from 86C to 61C. Express your answer to two significant figures and include the appropriate units. Calculate the kilocalories needed to heat 110g of water from 15C to 72C. Express your answer to two significant figures and include the appropriate units. Calculate the kilojoules needed to heat 165g of copper from 28C to 190C. Express your answer to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


