Question: An inventor develops a stationary cycling device by which an individual, while pedaling, can convert all of the energy expended into heat for warming water.
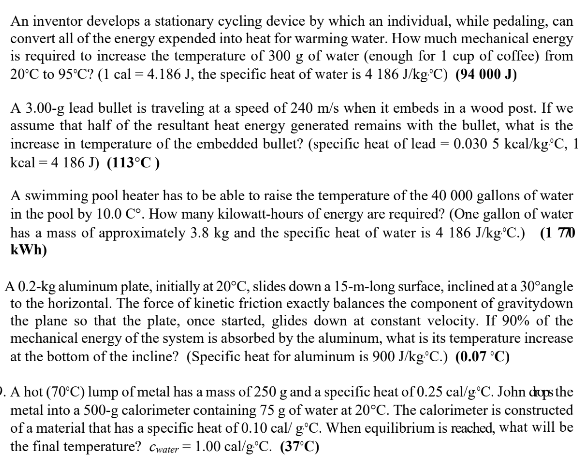
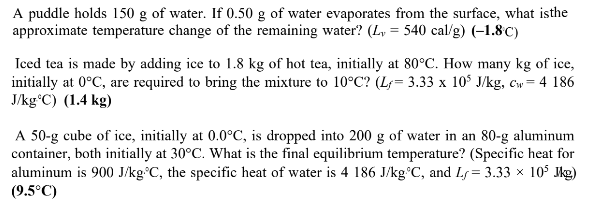

An inventor develops a stationary cycling device by which an individual, while pedaling, can convert all of the energy expended into heat for warming water. How much mechanical energy is required to increase the temperature of 300 g of water (enough for 1 cup of coffee) from 20'C to 95'C? (1 cal = 4.186 J. the specific heat of water is 4 186 J/kg C) (94 000 J) A 3.00-g lead bullet is traveling at a speed of 240 m's when it embeds in a wood post. If we assume that half of the resultant heat energy generated remains with the bullet, what is the increase in temperature of the embedded bullet? (specific heat of lead = 0.030 5 kcal/kg C, 1 kcal = 4 186 J) (113 C ) A swimming pool heater has to be able to raise the temperature of the 40 000 gallons of water in the pool by 10.0 Co. How many kilowatt-hours of energy are required? (One gallon of water has a mass of approximately 3.8 kg and the specific heat of water is 4 186 J/kg C.) (1 7/) k Wh) A 0.2-kg aluminum plate, initially at 20"C, slides down a 15-m-long surface, inclined at a 30"angle to the horizontal. The force of kinetic friction exactly balances the component of gravitydown the plane so that the plate, once started, glides down at constant velocity. If 90% of the mechanical energy of the system is absorbed by the aluminum, what is its temperature increase at the bottom of the incline? (Specific heat for aluminum is 900 J/kg C.) (0.07 *C) A hot (70'C) lump of metal has a mass of 250 g and a specific heat of 0.25 calig*C. John dups the metal into a 500-g calorimeter containing 75 g of water at 20 C. The calorimeter is constructed of a material that has a specific heat of 0.10 cal/ g'C. When equilibrium is reached, what will be the final temperature? Cwater = 1.00 cal/g.*C. (37:C)A puddle holds 150 g of water. If 0.50 g of water evaporates from the surface, what is the approximate temperature change of the remaining water? (L, = 540 cal/g) (-1.80) Iced tea is made by adding ice to 1.8 kg of hot tea, initially at 80 C. How many kg of ice, initially at 0'C, are required to bring the mixture to 10"C? (1/= 3.33 x 105 J/kg, Cw = 4 186 Jkg*C) (1.4 kg) A 50-g cube of ice, initially at 0.0"C, is dropped into 200 g of water in an 80-g aluminum container, both initially at 30 C. What is the final equilibrium temperature? (Specific heat for aluminum is 900 J/kg. C, the specific heat of water is 4 186 J/kg C, and Ly = 3.33 x 10' lig) (9.5'C)125 g of dry ice (solid CO2) is dropped into a beaker containing 500 g of 66 C water. The dry ice converts directly to gas, leaving the solution. When the dry ice is gone, the final temperature of the water is 29'C. What is the heat of vaporization of solid CO2? (Cwater = 1.00 cal/g'C) (148 cal/g) 100 g of liquid nitrogen at its boiling point of 77 K is stirred into a beaker containing 500 g of 15'C water. If the nitrogen leaves the solution as soon as it turns to gas, how much water freezes? The heat of vaporization of nitrogen is 48 cal/g and the heat of fusion of water is 80 cal/g. (none)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


