Question: Below are the concentration used in the lab. Please help me do mol fraction for the following table using the eqn given. 0.100 M Cu2+
Below are the concentration used in the lab. Please help me do mol fraction for the following table using the eqn given.
0.100 M Cu2+ 3.98 103 M Fe3+ 0.400 M Ni2+
0.100 M EDTA 3.98 103 M SCN 0.400 M en
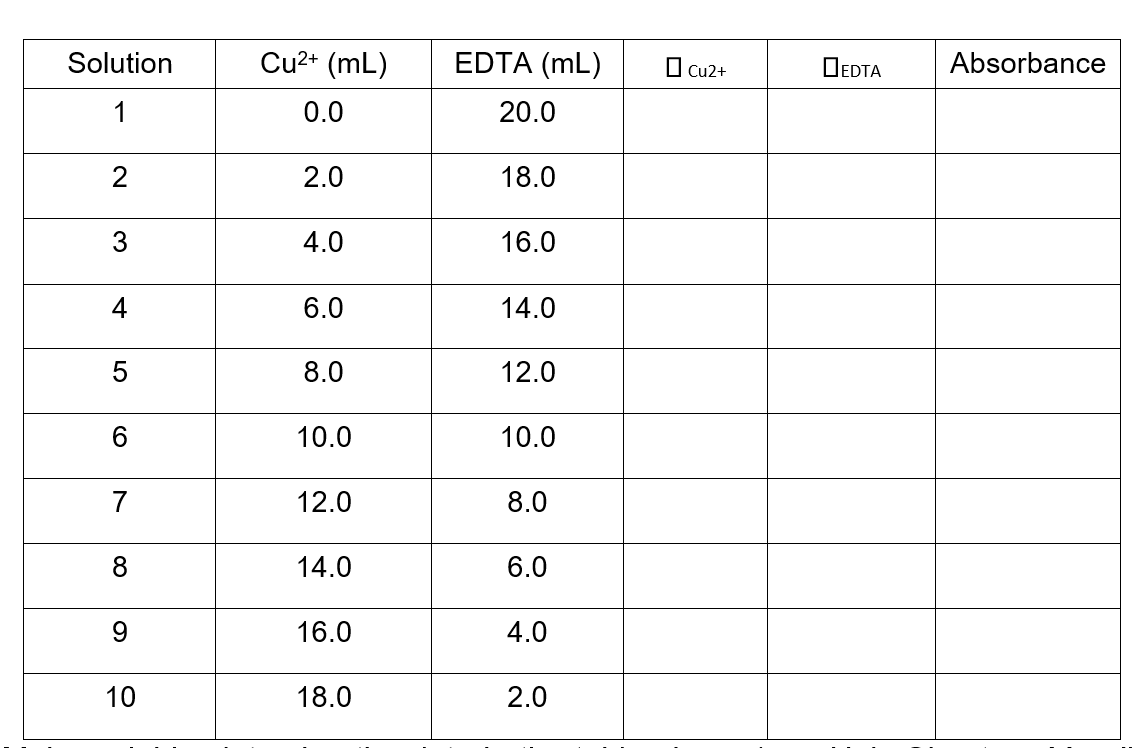
The mole fraction () is a ratio of the various species in solution. One of the solutions in this experiment is a two-component mixture of Cu2+ and EDTA.
The mole fraction for EDTA would therefore be expressed as: EDTA = (# mol EDTA) / (# mol EDTA + # mol Cu2+)
The mole fraction for copper(II) would then be: Cu2+ = 1 EDTA
Could you please do a sample calulation for me
 0.100 M Cu2+ 3.98 103 M Fe3+ 0.400 M Ni2+ 0.100 M EDTA 3.98 103 M SCN 0.400 M en The mole fraction () is a ratio of the various species in solution. One of the solutions in this experiment is a two-component mixture of Cu2+ and EDTA. The mole fraction for EDTA would therefore be expressed as: EDTA = (# mol EDTA) / (# mol EDTA + # mol Cu2+) The mole fraction for copper(II) would then be: Cu2+ = 1 EDTA
0.100 M Cu2+ 3.98 103 M Fe3+ 0.400 M Ni2+ 0.100 M EDTA 3.98 103 M SCN 0.400 M en The mole fraction () is a ratio of the various species in solution. One of the solutions in this experiment is a two-component mixture of Cu2+ and EDTA. The mole fraction for EDTA would therefore be expressed as: EDTA = (# mol EDTA) / (# mol EDTA + # mol Cu2+) The mole fraction for copper(II) would then be: Cu2+ = 1 EDTA
\begin{tabular}{|c|c|c|c|c|c|} \hline Solution & Cu 2+(mL) & EDTA (mL) & cu2+ & DEDTA & Absorbance \\ \hline 1 & 0.0 & 20.0 & & & \\ \hline 2 & 2.0 & 18.0 & & & \\ \hline 3 & 4.0 & 16.0 & & & \\ \hline 4 & 6.0 & 14.0 & & & \\ \hline 5 & 8.0 & 12.0 & & & \\ \hline 6 & 10.0 & 10.0 & & & \\ \hline 7 & 12.0 & 8.0 & & & \\ \hline 8 & 16.0 & 6.0 & & & \\ \hline 9 & 18.0 & 2.0 & & & \\ \hline 10 & & & & \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


