Question: Below is a pH curve that Harry plotted based on the the solution in the Erlenmeyer flask while he was carrying out titration in class.
Below is a pH curve that Harry plotted based on the the solution in the Erlenmeyer flask while he was carrying out titration in class. Which of the following statement is true.
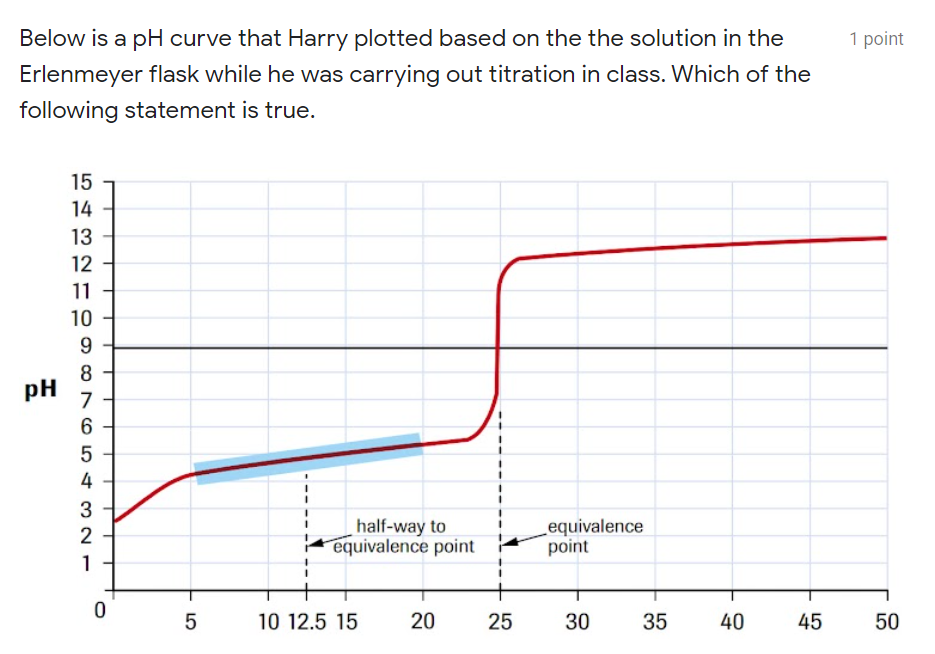
a) HCl(aq) from the burette is added to the NaOH(aq) in the Erlenmeyer flask.
b) NaOH(aq) from the burette is added to the HCl(aq) in the Erlenmeyer flask.
c) HCl(aq) from the burette is added to the NH3(aq) in the Erlenmeyer flask.
d) NH3(aq) from the burette is added to the HCl(aq) in the Erlenmeyer flask.
e) CH3COOH(aq) from the burette is added to the NaOH(aq) in the Erlenmeyer flask.
f) NaOH(aq) from the burette is added to the CH3COOH(aq) in the Erlenmeyer flask.
2) Based on the titration curve in the previous question, explain why there is a slight increase at the start of the graph (prior to reaching the first "plateau"), while some titration curves do not have such a slight increase. Explain your answer in not more than two sentences.
3) When using indicators we only use a very small amount. Often only a few drops. Why? What would happen if too much indicator is used? Explain your answer in not more than two sentences.
4) When Harry was carrying out titration in class, he added a few more drops of indicator than what Mr. Tse told him to. As a result, will he need to add a few more drops of base (than he originially had to) if he's trying to titrate the acid that has an unknown concentration? Explain your answer in not more than two sentences. *
1 point Below is a pH curve that Harry plotted based on the the solution in the Erlenmeyer flask while he was carrying out titration in class. Which of the following statement is true. 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 pH half-way to equivalence point equivalence point 0 5 10 12.5 15 20 25 30 35 40 45 50
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


