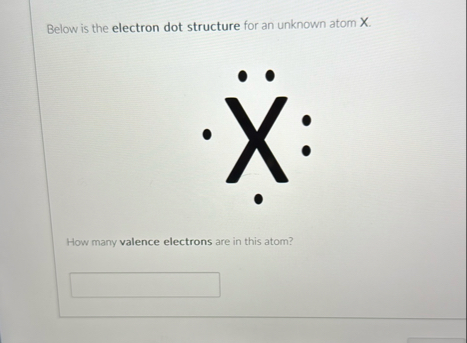
Question: Below is the electron dot structure for an unknown atom x . How many valence electrons are in this atom? You deposit $ 1 0
Below is the electron dot structure for an unknown atom
How many valence electrons are in this atom?
You deposit $ in an account that pays interest compounded quarterly.
a Find the future value ater one year.
b Use the future value formula for simple interest to determine the effective annual yield.
Click the icon to view some finance formulas.
a The future value is $
Round to the nearest cent as needed.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


