Question: Bicarbonate does buffer the blood because carbonic acid is generated from dissolving CO2(g) in liquid water: CO2(g)+H2O(I)H2CO3(aq)pKeq=2.52at37C This reaction is catalyzed by the enzyme carbonic

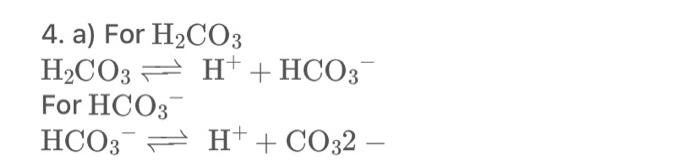

Bicarbonate does buffer the blood because carbonic acid is generated from dissolving CO2(g) in liquid water: CO2(g)+H2O(I)H2CO3(aq)pKeq=2.52at37C This reaction is catalyzed by the enzyme carbonic anhydrase. Consequently, the reaction that really represents what's happening when bicarbonate buffers the blood is: CO2(g)+H2O(I)HCO3(aq)+H+(aq) Calculate the equilibrium constant for this reaction (Hint: use one of the equilibrium expressions from question 4 above). Would you expect this to be an adequate buffer system at physiological pH? Explain. 4.a)ForH2CO3H2CO3H++HCO3ForHCO3HCO3H++CO32 Bicarbonate does buffer the blood because carbonic acid is generated from dissolving CO2(g) in liquid water: CO2(g)+H2O(I)H2CO3(aq)pKeq=2.52at37C This reaction is catalyzed by the enzyme carbonic anhydrase. Consequently, the reaction that really represents what's happening when bicarbonate buffers the blood is: CO2(g)+H2O(I)HCO3(aq)+H+(aq) Calculate the equilibrium constant for this reaction (Hint: use one of the equilibrium expressions from question 4a above). Would you expect this to be an adequate buffer system at physiological pH? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


