Question: please need help!! 25 Lab 3-pH and Buffers Results Name & Lab Section: A. The pH scale and the Determination of pH Substance pH paper
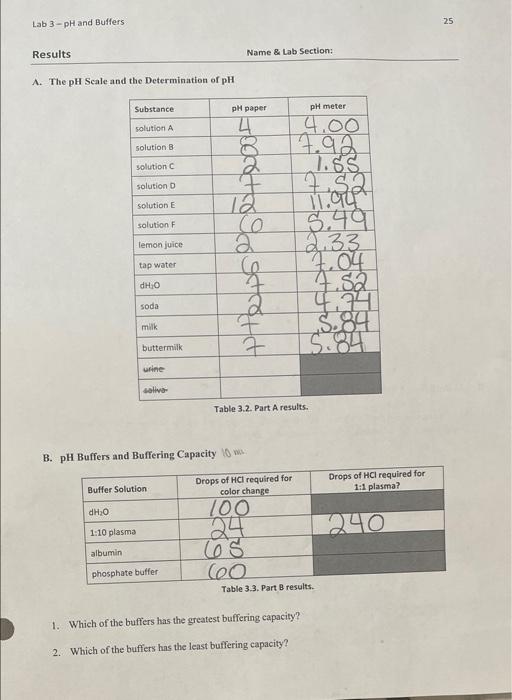

25 Lab 3-pH and Buffers Results Name & Lab Section: A. The pH scale and the Determination of pH Substance pH paper pH meter solution A solution B solution solution D solution 12 solution F woodstrand 4.00 17.92 1.85 Tate $ 49 2.33 7.52 4.4 5.89 5.84 lemon juice tap water 2004 soda milk buttermilk urine alive Table 3.2. Part A results. B. pH Buffers and Buffering Capacity Drops of HCl required for color change Drops of HCl required for 1:1 plasma? Buffer Solution dH20 240 1:10 plasma 100 24 608 6o albumin phosphate buffer Table 3.3. Part B results. 1. Which of the buffers has the greatest buffering capacity? 2. Which of the buffers has the least buffering capacity? 26 Lab 3-pH and Buffers Discussion A. The pH scale and the Determination of pH 1. How would you explain the variation in readings between the two methods? 2. Why does there seem to be a variation in pH of different students' urine samples? 3. Define pH B. pH Buffers and Buffering Capacity 1. Most metabolic end products are acidic. Therefore, in order for blood to be a better buffer, would blood contain more carbonic acid or bicarbonate? Explain. 2. List one example in the human body where change in pH affects physiology. Explain your example
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


