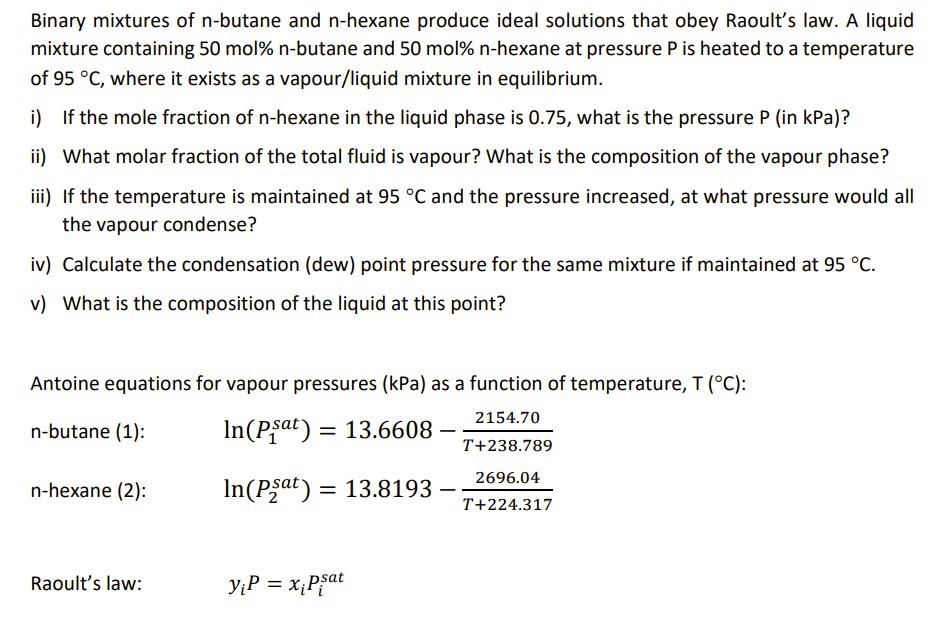
Question: Binary mixtures of n - butane and n - hexane produce ideal solutions that obey Raoult's law. A liquid mixture containing 5 0 mol %
Binary mixtures of butane and hexane produce ideal solutions that obey Raoult's law. A liquid
mixture containing molbutane and molhexane at pressure is heated to a temperature
of where it exists as a vapourliquid mixture in equilibrium.
i If the mole fraction of hexane in the liquid phase is what is the pressure in kPa
ii What molar fraction of the total fluid is vapour? What is the composition of the vapour phase?
iii If the temperature is maintained at and the pressure increased, at what pressure would all
the vapour condense?
iv Calculate the condensation dew point pressure for the same mixture if maintained at
v What is the composition of the liquid at this point?
Antoine equations for vapour pressures kPa as a function of temperature, :
nbutane :
nhexane :
Raoult's law:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


