Question: A crude fermenter for anaerobic fermentation is set up with ammonia as the nitrogen source. There are about 0.45 g of ethanol (C2H6O) are
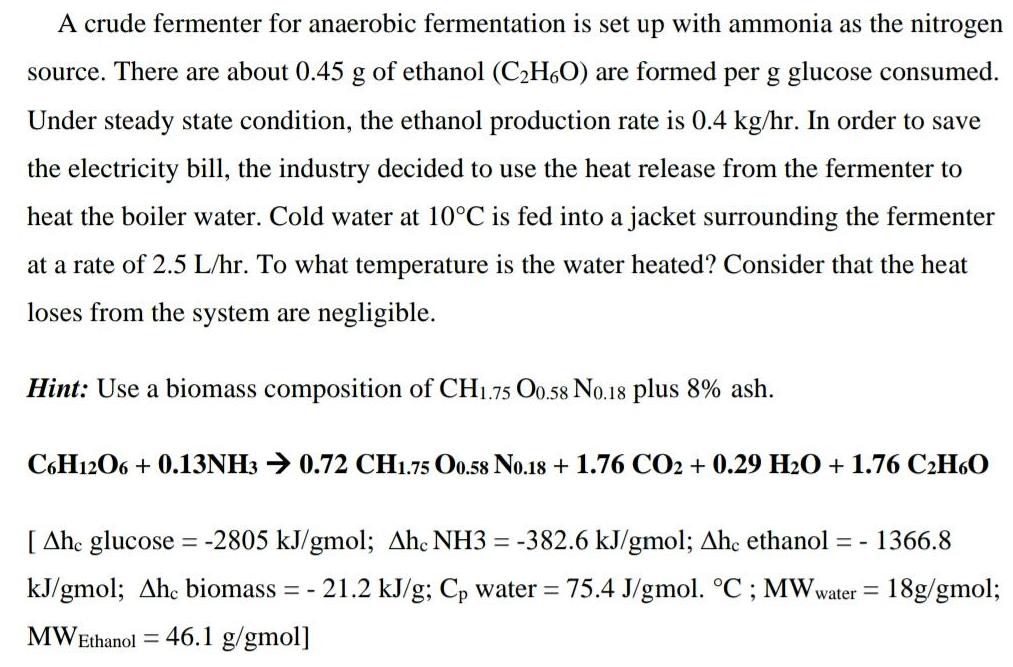
A crude fermenter for anaerobic fermentation is set up with ammonia as the nitrogen source. There are about 0.45 g of ethanol (C2H6O) are formed per g glucose consumed. Under steady state condition, the ethanol production rate is 0.4 kg/hr. In order to save the electricity bill, the industry decided to use the heat release from the fermenter to heat the boiler water. Cold water at 10C is fed into a jacket surrounding the fermenter at a rate of 2.5 L/hr. To what temperature is the water heated? Consider that the heat loses from the system are negligible. Hint: Use a biomass composition of CH1.75 Oo.58 No.18 plus 8% ash. C6H1206 + 0.13NH3 0.72 CH1.75 Oo.58 No.18 + 1.76 CO2 + 0.29 H2O + 1.76 C2H6O [ Ahe glucose = -2805 kJ/gmol; Ahc NH3 = -382.6 kJ/gmol; Ahe ethanol = - 1366.8 kJ/gmol; Ahc biomass 21.2 kJ/g; Cp water = 75.4 J/gmol. C ; MWwater = 18g/gmol; = - MWEthanol 46.1 g/gmol]
Step by Step Solution
3.46 Rating (146 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


