Question: 1. Quantities of natural gas are measured in normal cubic meters at 0C (273.15 K) and 101.325 kPa or in standard cubic feet at
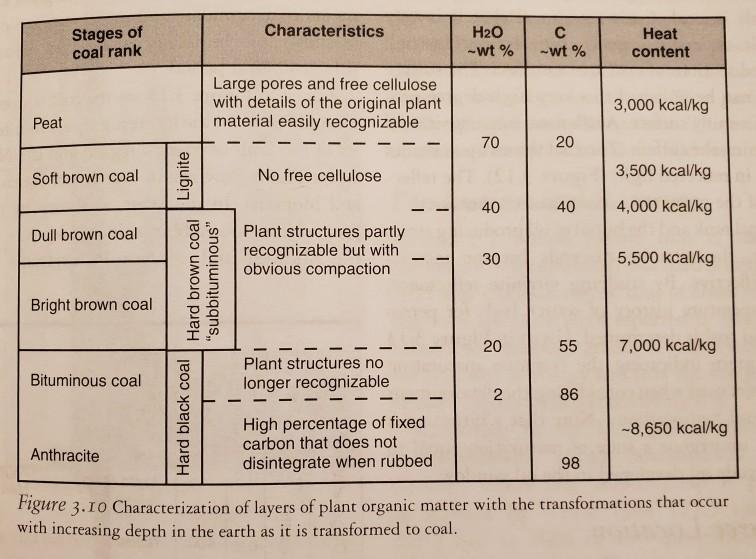
1. Quantities of natural gas are measured in normal cubic meters at 0C (273.15 K) and 101.325 kPa or in standard cubic feet at 60F (288.75 K) and 14.73 psi (= pounds per square inch). If natural behaves as an ideal gas (PV nRT) where n is the number gas of moles, what is the conversion factor between the two units? %3D Show your work (1 psi = 6,894 Pa, 1 foot = 0.3048 meters, and R = 8.31424 J mol- K-). 2. a. Calculate the heat released in Btu from 1 metric ton of lignite and 1 metric ton of anthracite as given in Figure 3.10 (1 Btu = 252 calories). b. If 127.5 quadrillion (10) Btu of coal energy was used in 2008 and on average this was brown coal. How many billion metric %3! tons of coal was used? Stages of coal rank Characteristics H20 -wt % Heat -wt % content Large pores and free cellulose with details of the original plant material easily recognizable 3,000 kcal/kg Peat 70 Soft brown coal No free cellulose 3,500 kcal/kg 40 40 4,000 kcal/kg Plant structures partly recognizable but with obvious compaction Dull brown coal 30 5,500 kcal/kg Bright brown coal 55 7,000 kcal/kg Plant structures no Bituminous coal longer recognizable 86 High percentage of fixed carbon that does not -8,650 kcal/kg Anthracite disintegrate when rubbed 98 Figure 3.10 Characterization of layers of plant organic matter with the transformations that occur with increasing depth in the earth as it is transformed to coal. Hard black coal Lignite Hard brown coal "subbituminous" 2. 20 20
Step by Step Solution
3.34 Rating (148 Votes )
There are 3 Steps involved in it
Q1 Q 2 a Ans To calculate heat released in Btu from one metric ton of Lignite we have to first conve... View full answer

Get step-by-step solutions from verified subject matter experts


