Question: Bond angle in SO3 molecule is it is molecule, and it is in BrF; molecule, respective 120, 90 109.50 120, 180, 90 109.50, 1800, 1200

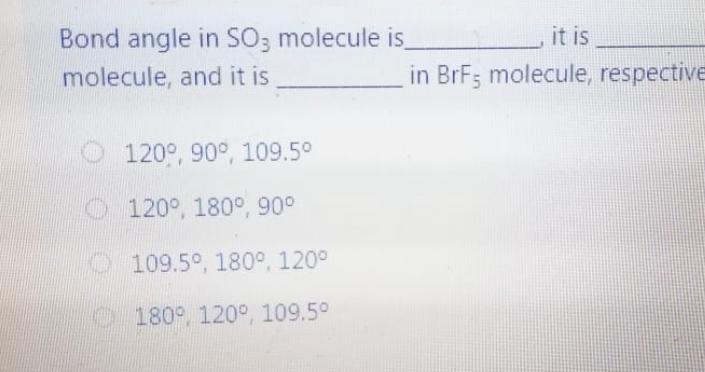
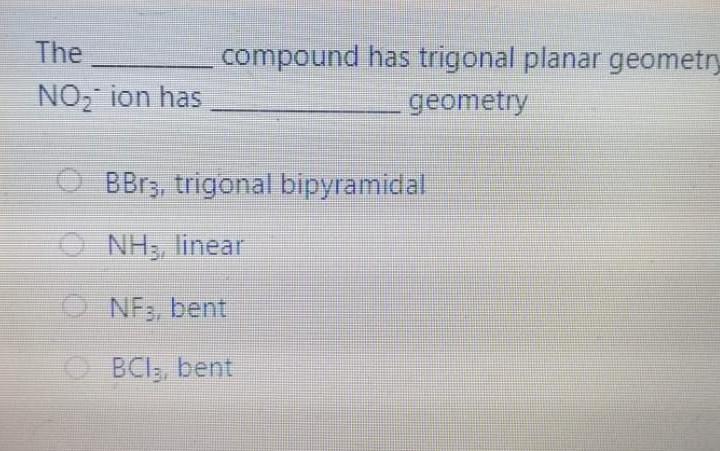





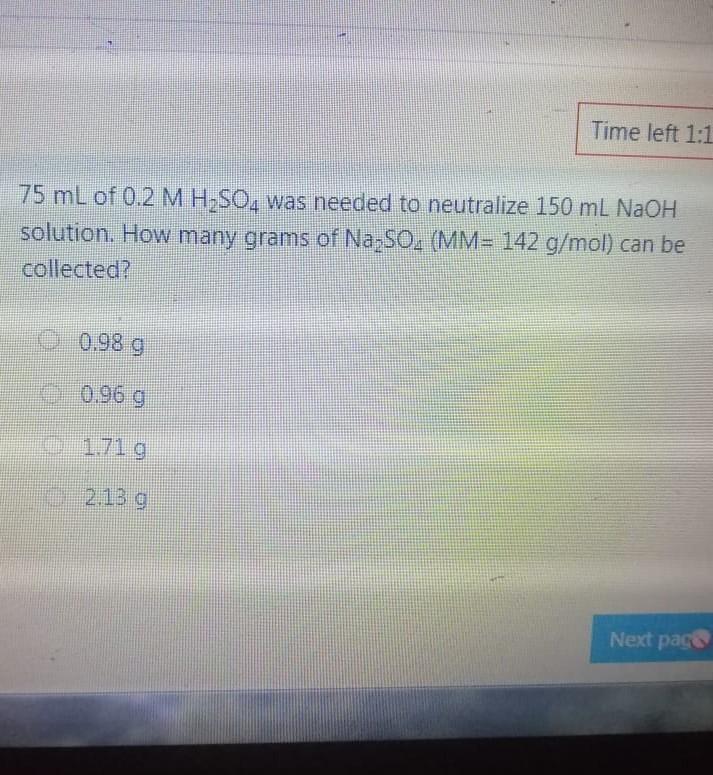
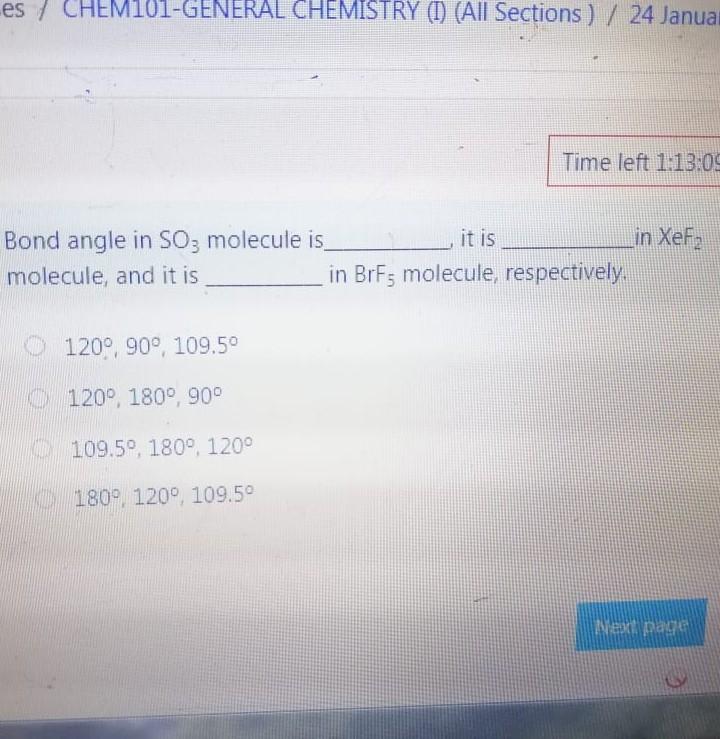
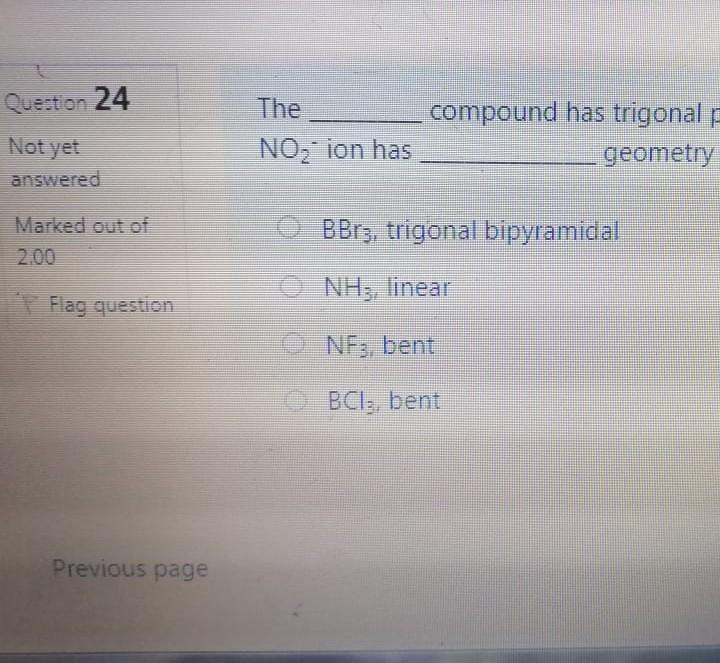
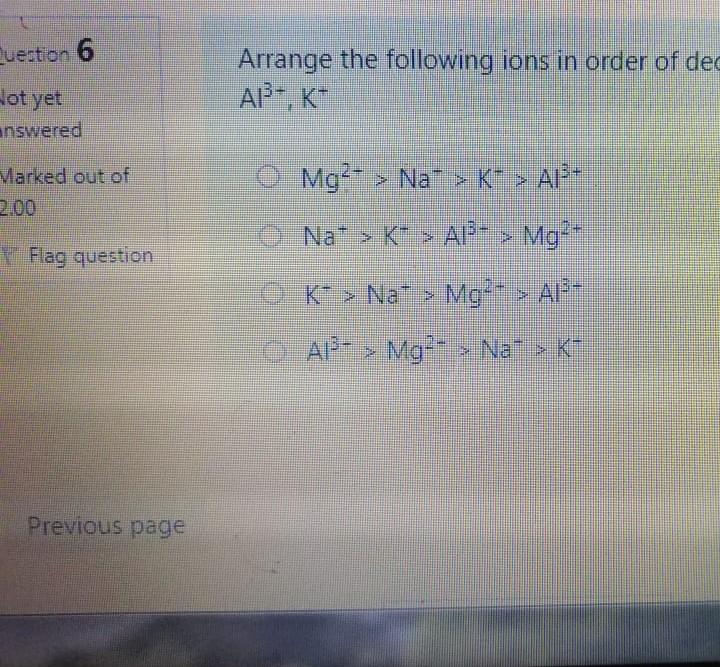
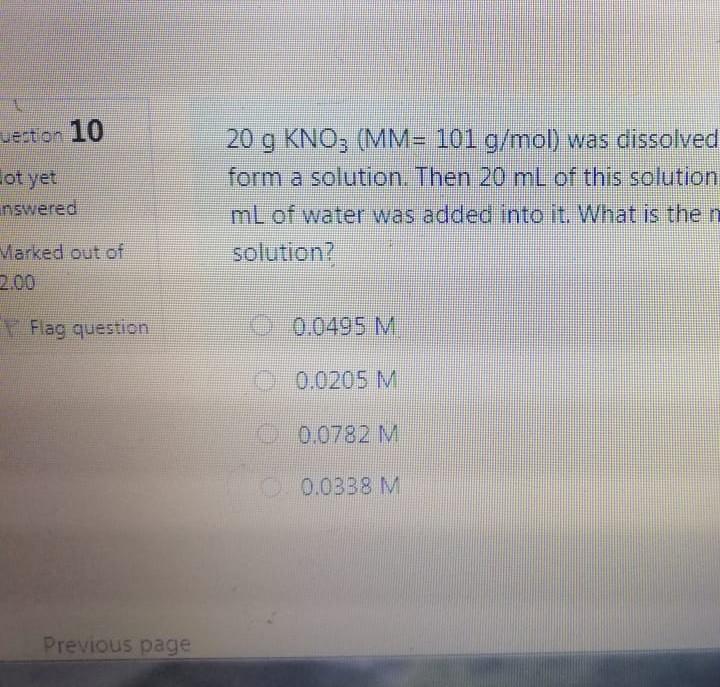
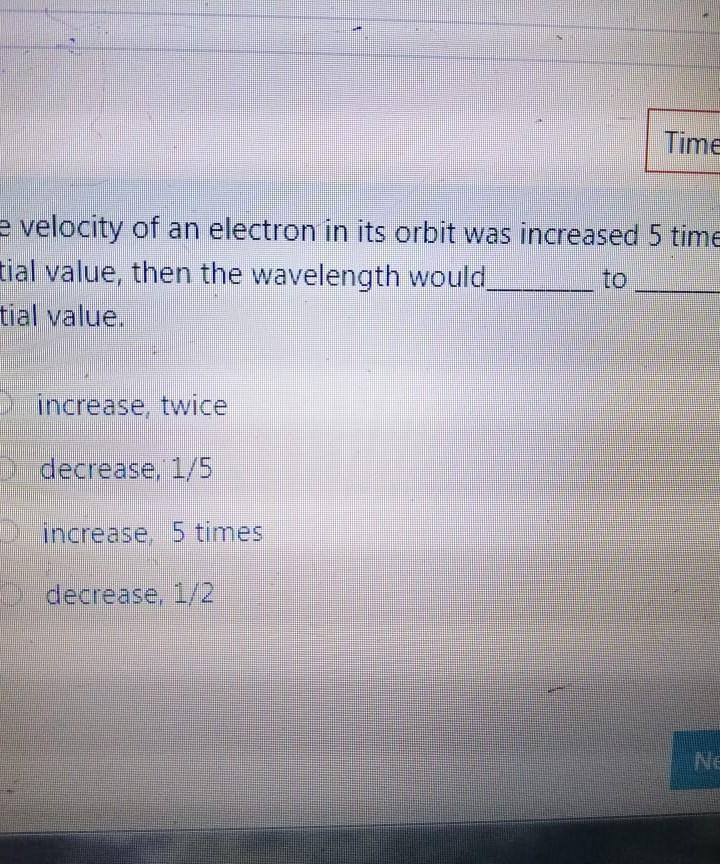
Bond angle in SO3 molecule is it is molecule, and it is in BrF; molecule, respective 120, 90 109.50 120, 180, 90 109.50, 1800, 1200 180 120 109.50 The NO, ion has compound has trigonal planar geometr geometry O BBr3, trigonal bipyramidal NH3, linear NE;, bent BC13, bent Which of the following sub-orbitals can never exist? 3d 1 4f 7s Arrange the followings in order of decreasing bond order B2, C2+, N2, F2 F2+ > B2 > C2+ > N2 O Czt > N2 > F2+ > B2 N2 > C2+ > B2 > F2 B2 > C2+ > N2 > F2+ 100 mL of 0.2 M H2SO4 was needed to neutralize 150 ml NaOH solution. How many grams of Na2SO4 (MM= 142 g/mol) can be collected? O 1.06 g O 3.77 g O 2.84 g 1.73 g The compound has square planar geometry, whereas the CIF3 compound has geometry PC15, trigonal pyramidal O XeF4, T-shaped O CCl4, tetrahedral O SF4, trigonal planar Marked out of 2.00 Consider the following apparatus. Calculate the partial pressures of helium and neon after the stopcock is open. The temperature remains constant at 16 C. He Ne 1.2 L 3.4 L 9.63 atm 2.8 atm Partial pressures: He = 0.18 atm; Ne = 1.4 atm Partial pressures: He = 0.22 atm; Ne = 0.61 atm Partial pressures: He = 0.16 atm; Ne = 2.1 atm Partial pressures: He = 0.24 atm; Ne = 2.3 atm Time left 1:1 75 mL of 0.2 M H:50. was needed to neutralize 150 ml NaOH solution. How many grams of Na2SO. (MM= 142 g/mol) can be collected? 0.98 g 0.96 9 1.719 Next page Les / CHEM101-GENERAL CHEMISTRY (1) (All Sections ) / 24 Januar Time left 113:09 Bond angle in SO3 molecule is it is in Xefa molecule, and it is in BrFs molecule, respectively. 120, 90 109.50 NO 120 1800 900 109.50 180 1200 180 1200, 109.5 Nex page Queston 24 The compound has trigonalp NO, ion has geometry Not yet answered Marked out of BBrz, trigonal bipyramidal NH;, linear Flag question NF; bent BCl3, bent Previous page Question 6 Arrange the following ions in order of de AP-, K Not yet inswered Marked out of 2.00 O Mg?- > Na > K-> AP- O Na' > K > AP-> Mg2- + Flag question OK" > Na+ > Mg2+ > AP- AB-> Mg-- > Na-> K- Previous page vestion 10 ot yet nswered 20 g KNO3 (MM= 101 g/mol) was dissolved form a solution. Then 20 mL of this solution mL of water was added into it. What is the n solution? Varked out of 2.00 Flag question 0.0495 M 0.0205 M 0.0782 M 0.0338 M Previous page Time e velocity of an electron in its orbit was increased 5 time tial value, then the wavelength would tial value. increase, twice decrease, 1/5 increase 5 times > decrease, 1/2 NE
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


