Question: Bonus Problem ( 10 points, the answer must be absolutely correct, not necessary to solve at all, Ch. 12). Acetic acid ionizes in aqueous solutions
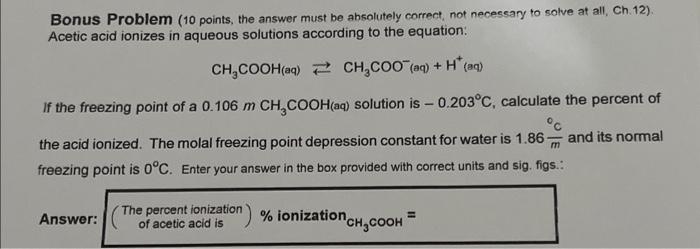
Bonus Problem ( 10 points, the answer must be absolutely correct, not necessary to solve at all, Ch. 12). Acetic acid ionizes in aqueous solutions according to the equation: CH3COOH(aq)CH3COO(aq)+H+(aq) If the freezing point of a 0.106mCH3COOH (aq) solution is 0.203C, calculate the percent of the acid ionized. The molal freezing point depression constant for water is 1.86mC and its normal freezing point is 0C. Enter your answer in the box provided with correct units and sig. figs.: Answer: ( Thepercentionizationofaceticacidis)% ionization CH3COOH=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


