Question: Bonus Problem ( +5pts). Consider the CSTR system shown in Figure 3. Stream 1 is a mixture of A and B with composition cA1 and
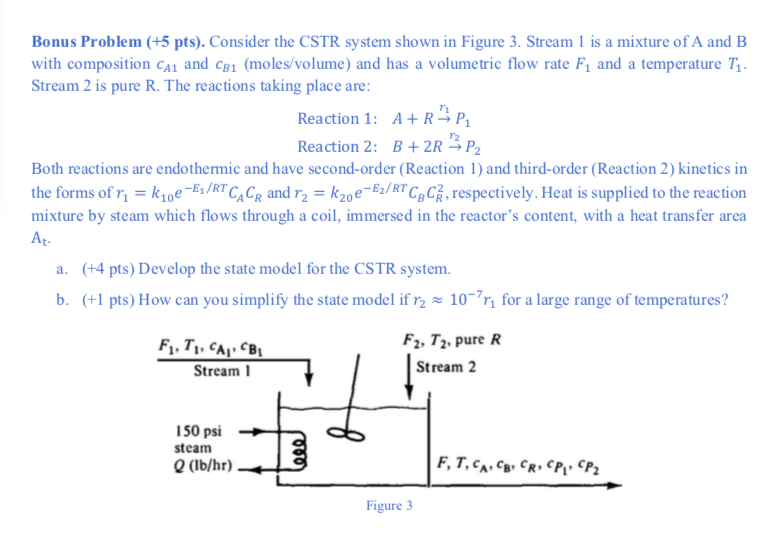
Bonus Problem ( +5pts). Consider the CSTR system shown in Figure 3. Stream 1 is a mixture of A and B with composition cA1 and cB1 (moles/volume) and has a volumetric flow rate F1 and a temperature T1. Stream 2 is pure R. The reactions taking place are: Reaction 1: A+Rr1P1 Reaction 2: B+2Rr2P2 Both reactions are endothermic and have second-order (Reaction 1) and third-order (Reaction 2) kinetics in the forms of r1=k10eE1/RTCACR and r2=k20eE2/RTCBCR2, respectively. Heat is supplied to the reaction mixture by steam which flows through a coil, immersed in the reactor's content, with a heat transfer area At. a. ( +4 pts) Develop the state model for the CSTR system. b. (+1 pts) How can you simplify the state model if r2107r1 for a large range of temperatures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


