Question: both parts pls will like Part A Identify the atom with the following orbital-filling diagram. 13252 2p 352 3p Express your answer as a chemical
both parts pls will like 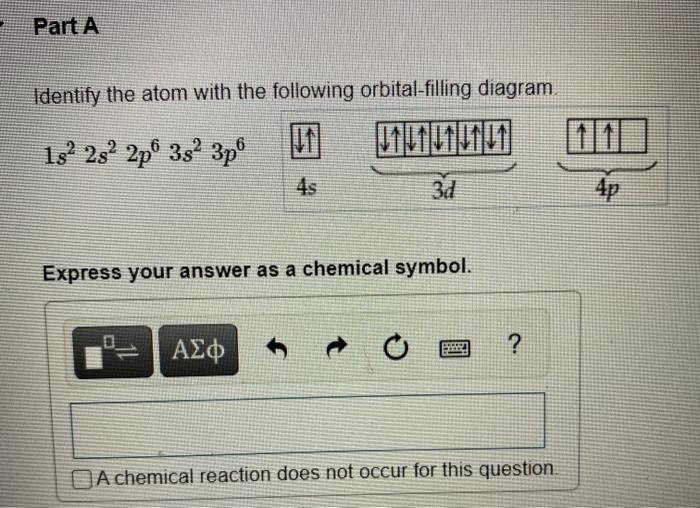

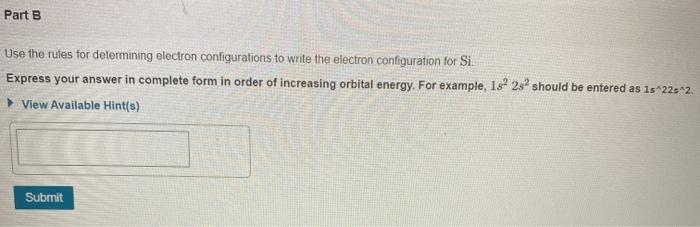
Part A Identify the atom with the following orbital-filling diagram. 13252 2p 352 3p Express your answer as a chemical symbol. ? O A chemical reaction does not occur for this question. Part B Use the rules for determining electron configurations to write the electron configuration for Si. Express your answer in complete form in order of increasing orbital energy. For example, 1s 2s should be entered as 15-225*2. View Available Hint(s) Submit
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


