Question: briefly explain why the relation shown by graph 3 exists in terms of the intermolecular forces involved begin{tabular}{|c|c|c|c|c|} hline Alcohol & methanol & ethanol &
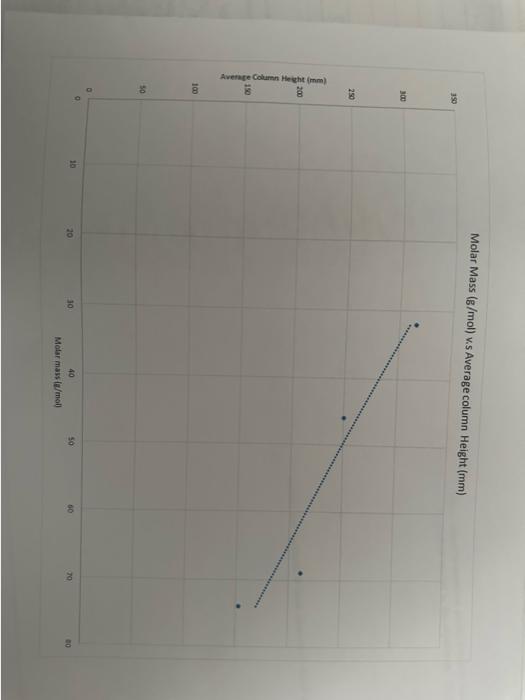
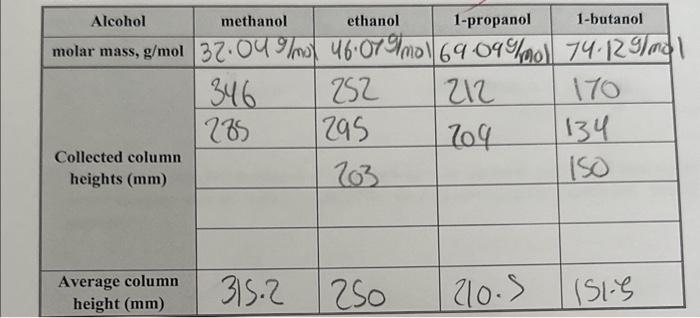
\begin{tabular}{|c|c|c|c|c|} \hline Alcohol & methanol & ethanol & 1-propanol & 1-butanol \\ \hline \multirow{4}{*}{molarmass,g/mol} & 32.049/mo & 46.079/mol & 69.099/mol & 74.129/mo \\ \hline \multirow{4}{*}{Collectedcolumnheights(mm)} & 346 & 252 & 212 & 170 \\ \cline { 2 - 5 } & 285 & 295 & 709 & 134 \\ \cline { 2 - 5 } & & 203 & 150 \\ \cline { 2 - 5 } & & & & \\ \hline \multirow{4}{*}{Averagecolumnheight(mm)} & 315.2 & 250 & 210.5 & 151.5 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


