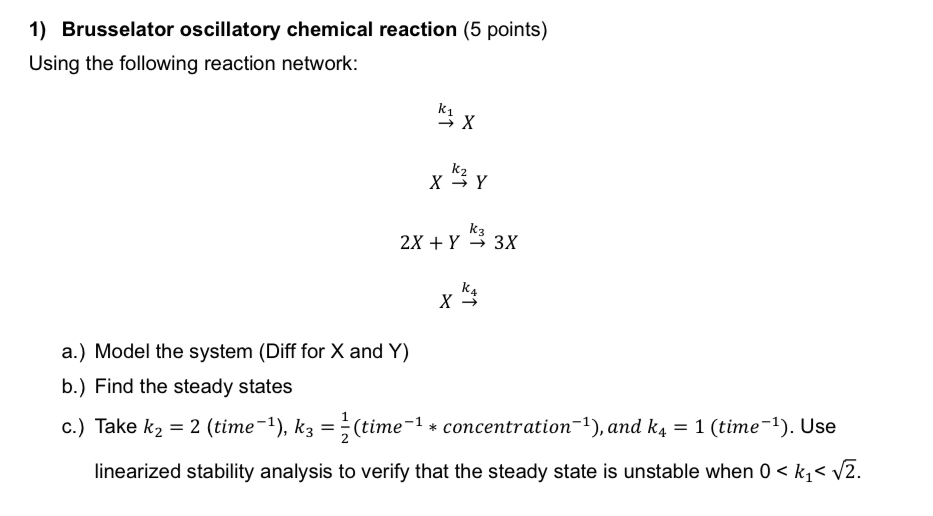
Question: Brusselator oscillatory chemical reaction ( 5 points ) Using the following reaction network: ? k 1 x x k 2 Y 2 x + Y
Brusselator oscillatory chemical reaction points
Using the following reaction network:
a Model the system Diff for and
b Find the steady states
c Take time time concentration : and time : Use linearized stability analysis to verify that the steady state is unstable when
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


