Question: Buffer solutions can be produced by mixing a weak acid with its conjugate base or by mixing a weak base with its conjugate acid. The
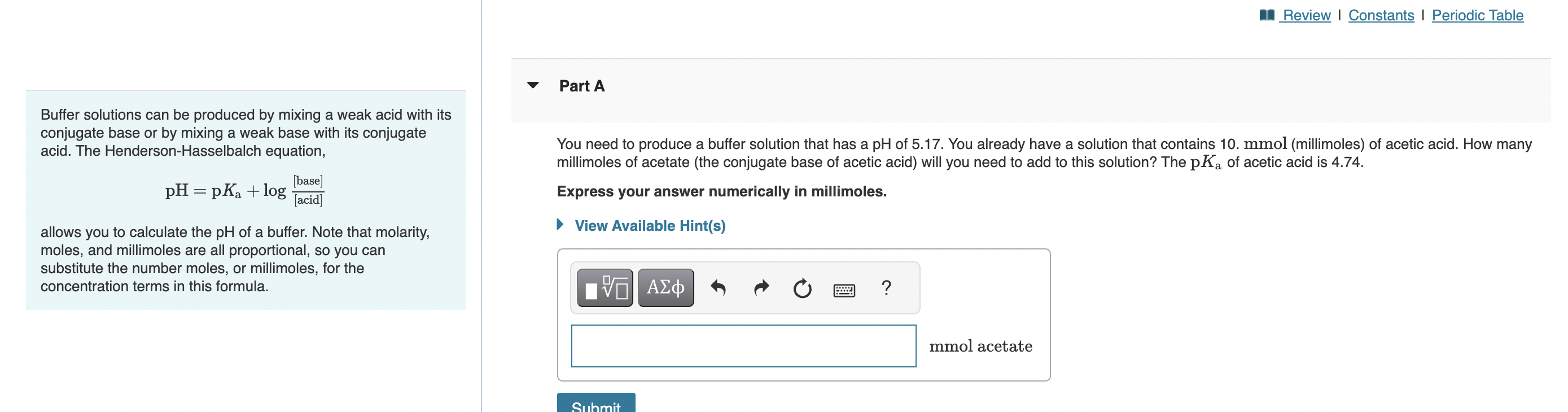
Buffer solutions can be produced by mixing a weak acid with its conjugate base or by mixing a weak base with its conjugate acid. The Henderson-Hasselbalch equation, You need to produce a buffer solution that has a pH of 5.17. You already have a solution that contains 10. mmol (millimoles) of acetic acid. How many millimoles of acetate (the conjugate base of acetic acid) will you need to add to this solution? The pKa of acetic acid is 4.74. pH=pKa+log[acid][base] Express your answer numerically in millimoles. allows you to calculate the pH of a buffer. Note that molarity, moles, and millimoles are all proportional, so you can substitute the number moles, or millimoles, for the concentration terms in this formula
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


