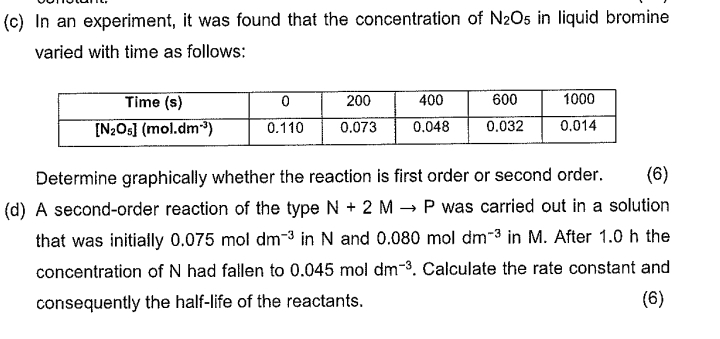
Question: ( c ) In an experiment, it was found that the concentration of N 2 O 5 in liquid bromine varied with time as follows:
c In an experiment, it was found that the concentration of in liquid bromine varied with time as follows:
tableTime s
Determine graphically whether the reaction is first order or second order.
d A secondorder reaction of the type was carried out in a solution that was initially in and in After the concentration of had fallen to Calculate the rate constant and consequently the halflife of the reactants.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


