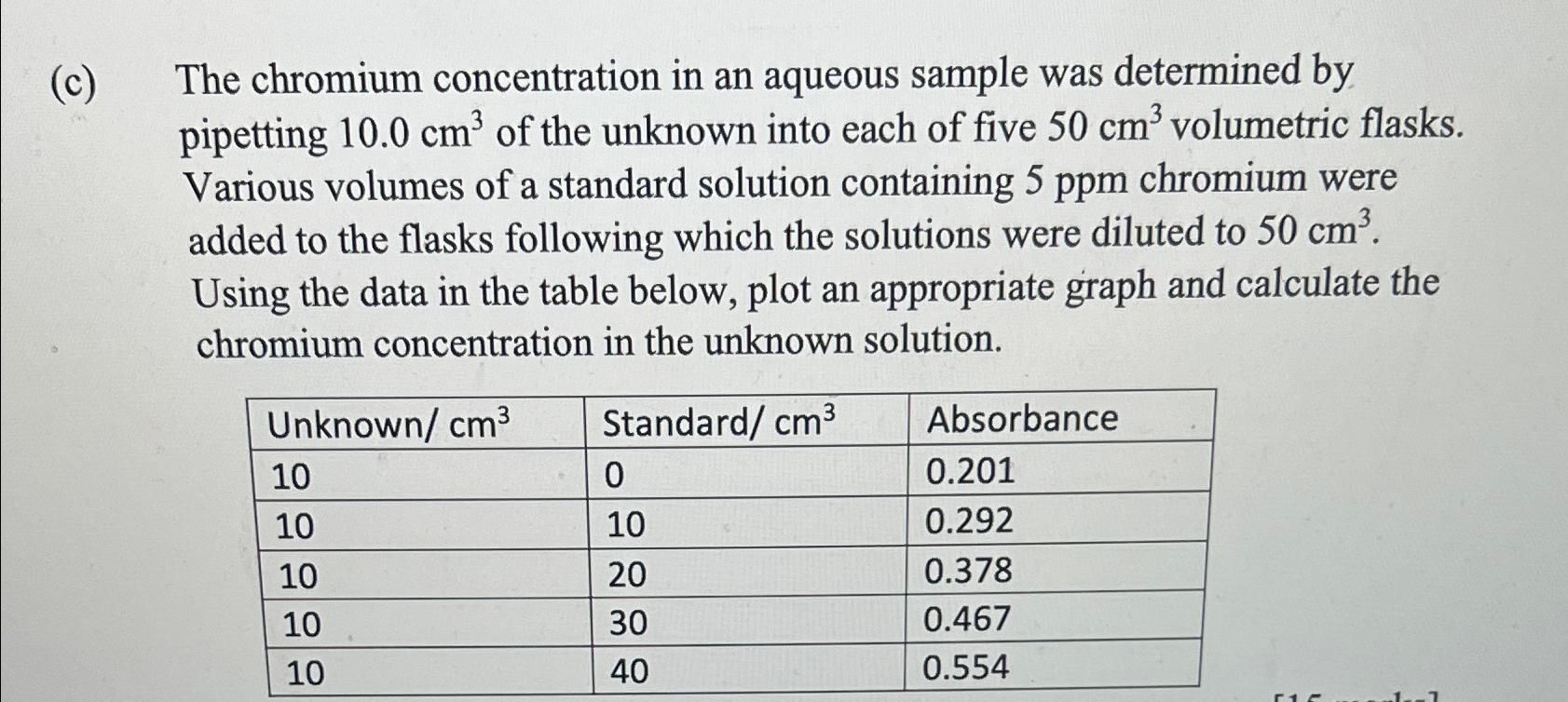
Question: ( c ) The chromium concentration in an aqueous sample was determined by pipetting 1 0 . 0 c m 3 of the unknown into
c The chromium concentration in an aqueous sample was determined by pipetting of the unknown into each of five volumetric flasks. Various volumes of a standard solution containing chromium were added to the flasks following which the solutions were diluted to Using the data in the table below, plot an appropriate graph and calculate the chromium concentration in the unknown solution.
tableUnknown Standard Absorbance
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


