Question: c. The van Deemter plot to the right shows the band broadening for three columns with different size stationary phase particles (1.8 ( mu mathrm{m},
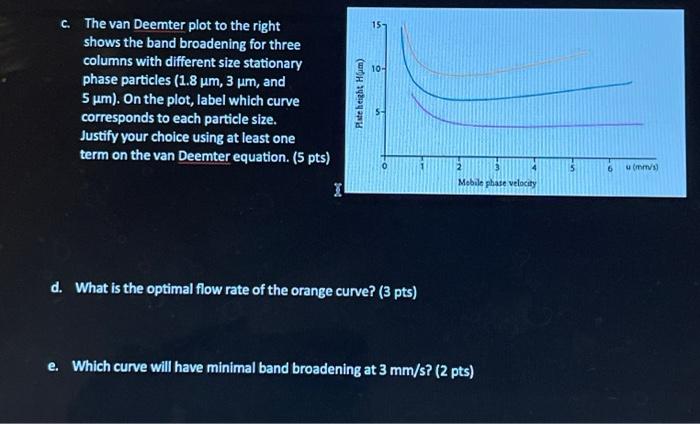
c. The van Deemter plot to the right shows the band broadening for three columns with different size stationary phase particles ( 1.8m,3m, and 5m). On the plot, label which curve corresponds to each particle size. Justify your choice using at least one term on the van Deemter equation. (5 pts) d. What is the optimal flow rate of the orange curve? (3 pts) e. Which curve will have minimal band broadening at 3mm/s ? (2 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


