Question: (b) Determine the atomic orbitals of the indium atom that can used to form o hybrid orbitals for the InCl2 (C4) ion. (6 marks)
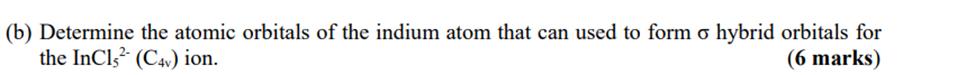
(b) Determine the atomic orbitals of the indium atom that can used to form o hybrid orbitals for the InCl2 (C4) ion. (6 marks)
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it
The atomic orbitals of the indium atom that can be used to form sigma hybrid orbitals for the InCls ... View full answer

Get step-by-step solutions from verified subject matter experts


