Question: calculate DAB by starting with the value in Table J.1 and then adjusting to the system temperature by assuming a simple T1.75 dependence. You should
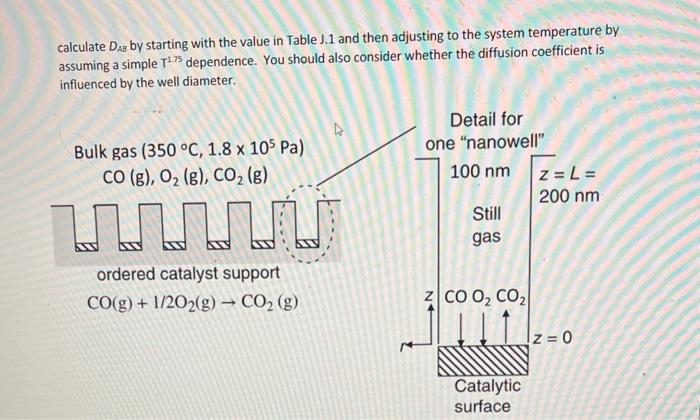

calculate DAB by starting with the value in Table J.1 and then adjusting to the system temperature by assuming a simple T1.75 dependence. You should also consider whether the diffusion coefficient is influenced by the well diameter. 2. Consider the novel "nanostructured" catalyst surface shown in the figure below. The catalyst support consists of an ordered array of 104 cylindrical "nanowells" of 100nm diameter and 200nm depth. A catalytic surface coats the bottom of each well. Although gas flows over the catalyst surface, the gas space within each "well" is stagnant - i.e., it is not well mixed. In the present application, the catalyst surface is used to convert CO gas (species A) and O2 gas (species B) into CO2 gas (species C ) according to the reaction CO(g)+1/2O2(g)CO2(g). The reaction is considered diffusion limited within the catalyst well. The process is isothermal at 350C and isobaric at 1.8x 105Pa bar total system pressure, with the bulk gas mole fraction compositions of yA=0.005,yB,= 0.99,yc=0.005. Calculate the rate at which CO is removed from the gas in g/hr. You should
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


