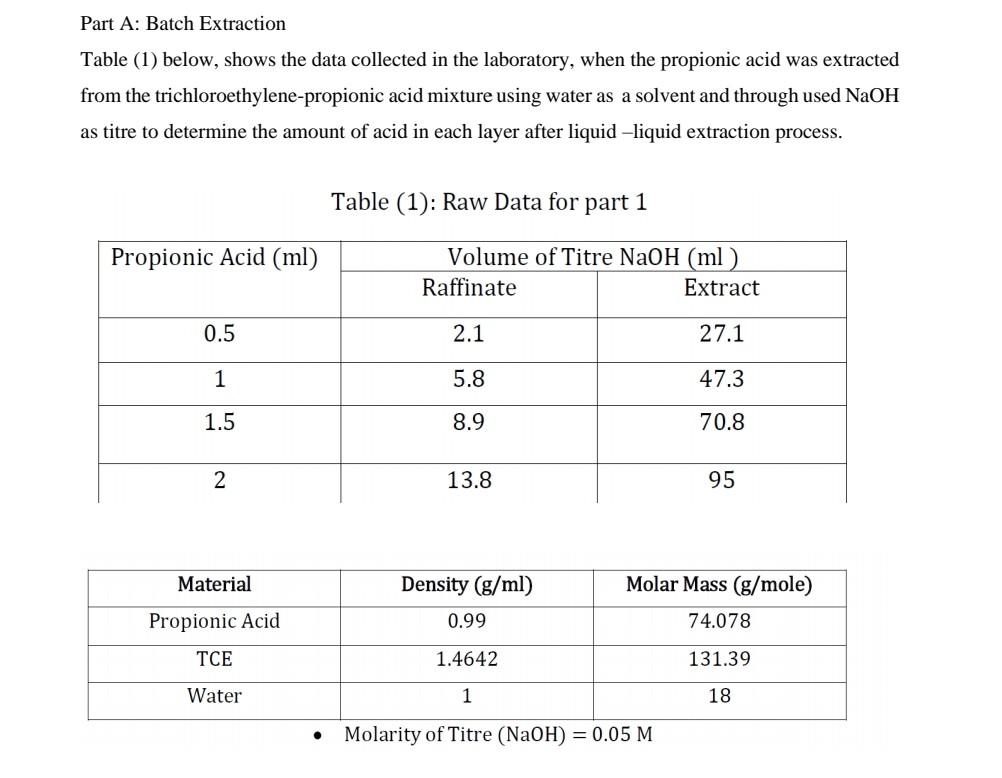
Question: calculate k Part A: Batch Extraction Table (1) below, shows the data collected in the laboratory, when the propionic acid was extracted from the trichloroethylene-propionic

calculate k
Part A: Batch Extraction Table (1) below, shows the data collected in the laboratory, when the propionic acid was extracted from the trichloroethylene-propionic acid mixture using water as a solvent and through used NaOH as titre to determine the amount of acid in each layer after liquid-liquid extraction process. Table (1): Raw Data for part 1 - Molarity of Titre (NaOH)=0.05M K=concentrationofsoluteinraffinatelayerconcentrationofsoluteinextractlayer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


