Question: Part A Data Time-temperatue data for Part A Run 1 Run 2 Molarity of HCl solution (M) 2.054 2.054 Volume of HCl solution (mL) 50.4
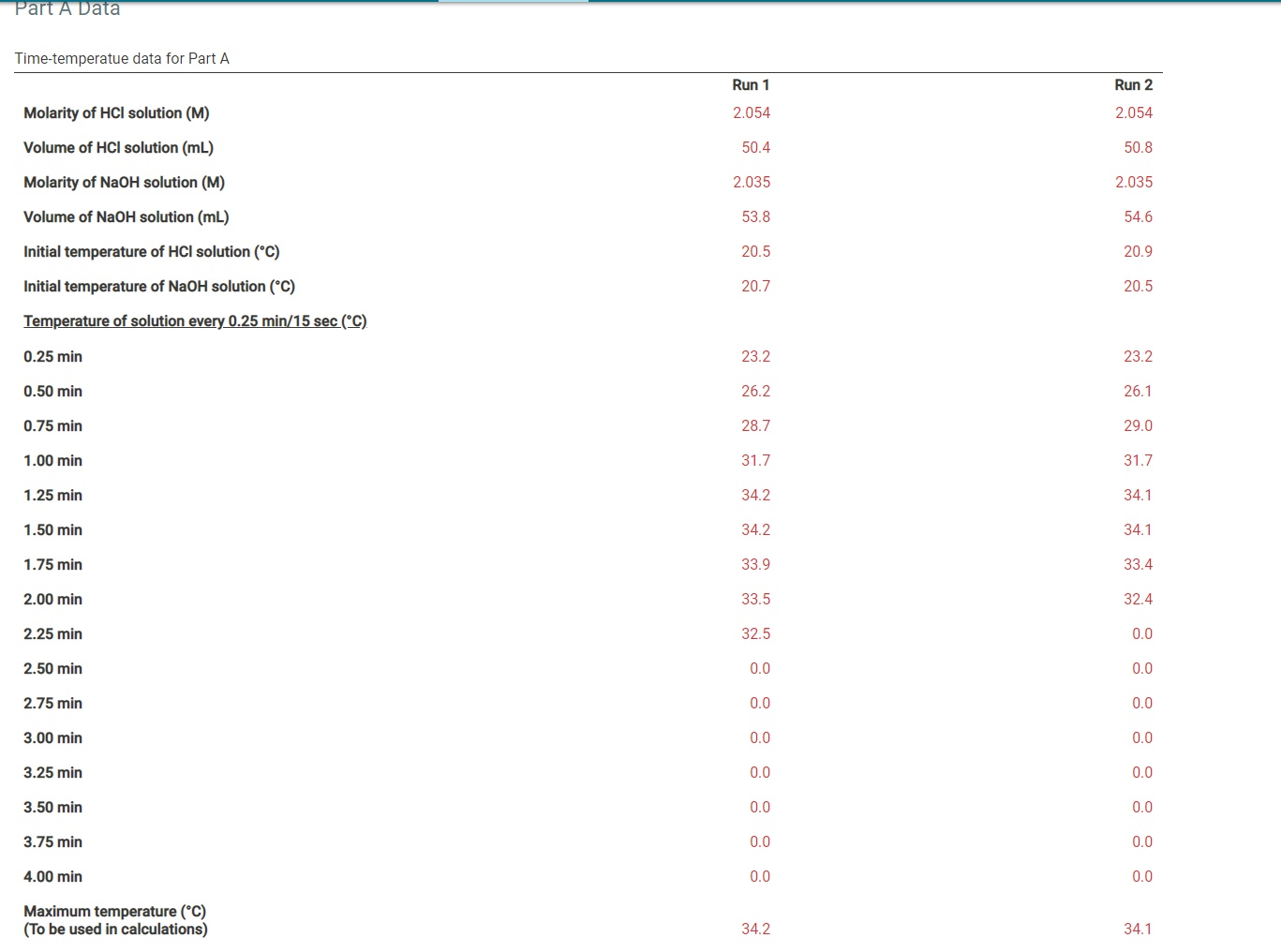
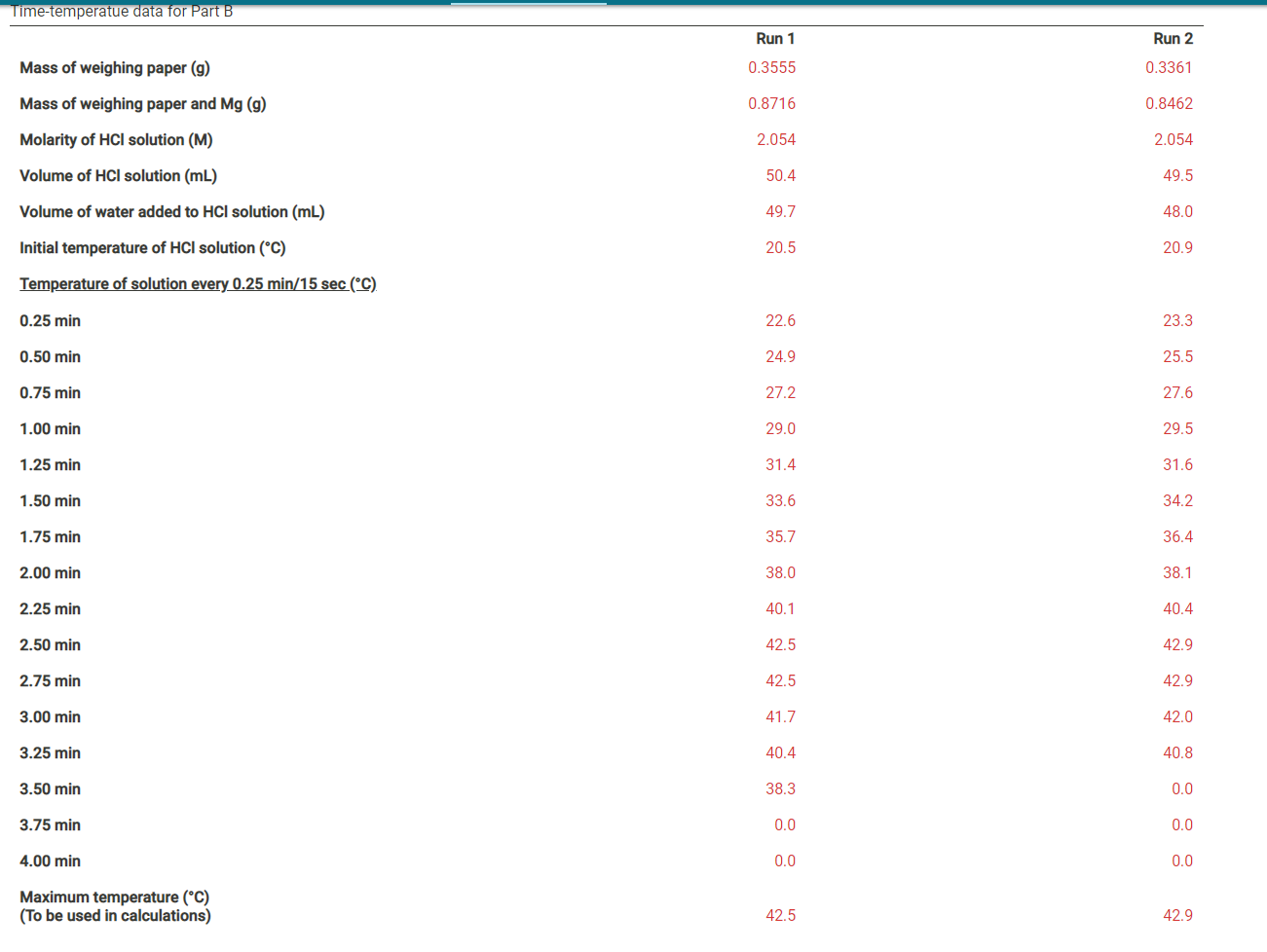
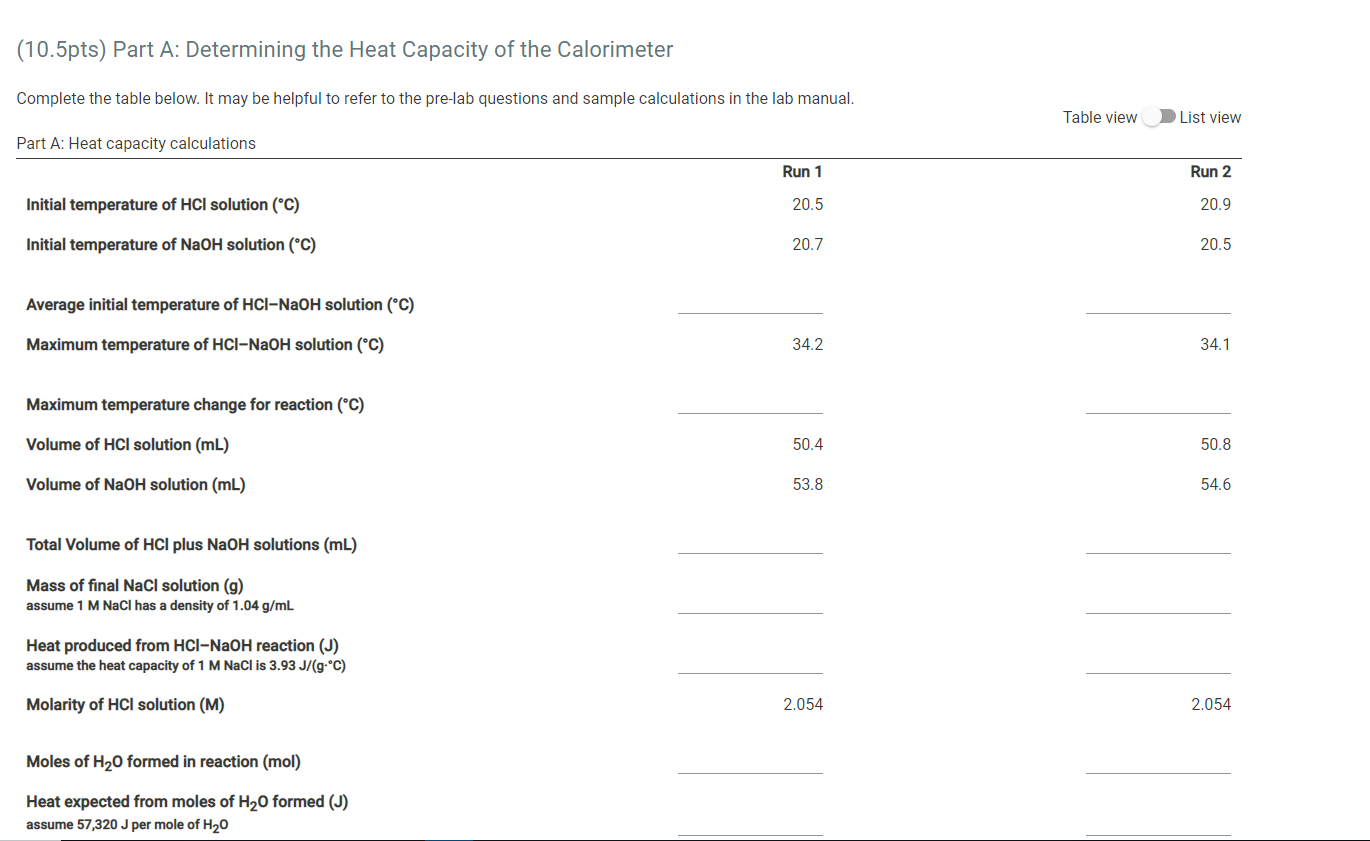


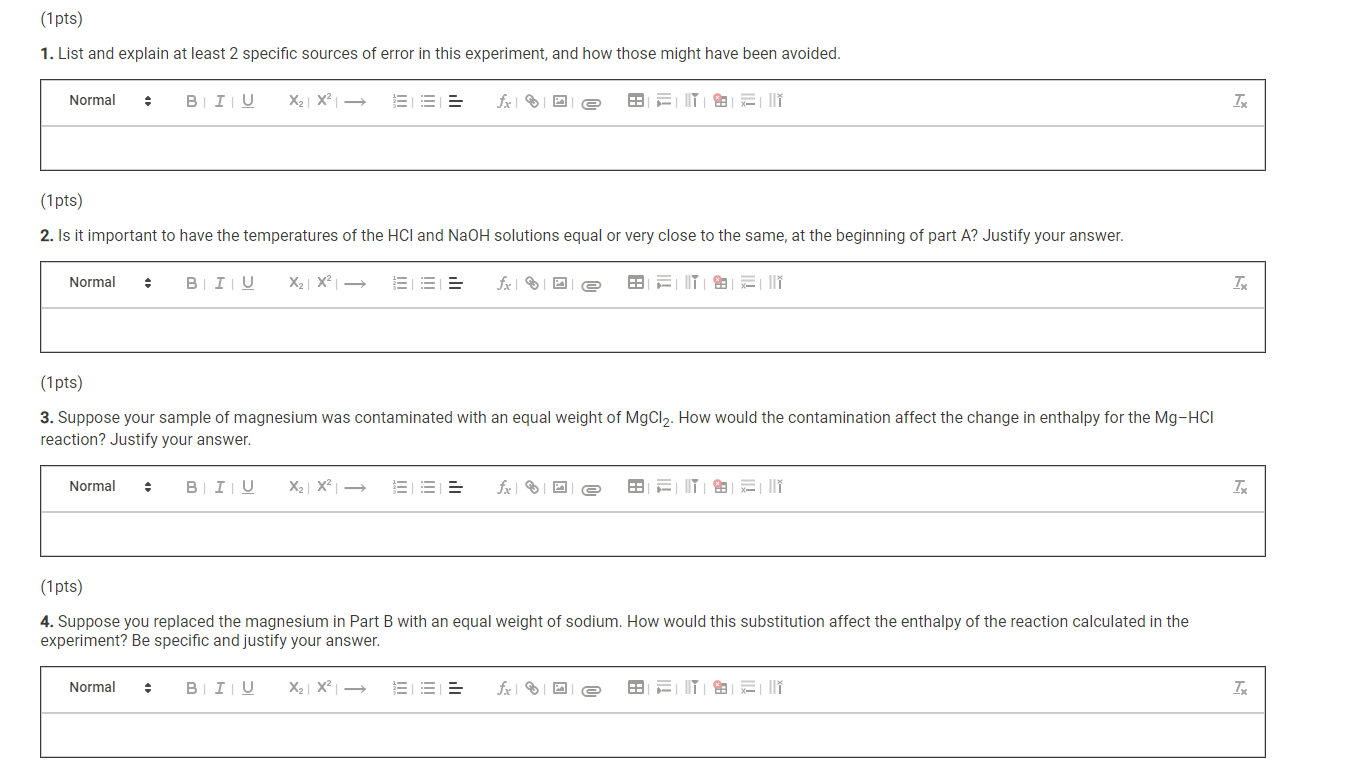
Part A Data Time-temperatue data for Part A Run 1 Run 2 Molarity of HCl solution (M) 2.054 2.054 Volume of HCl solution (mL) 50.4 50.8 Molarity of NaOH solution (M) 2.035 2.035 Volume of NaOH solution (mL) 53.8 54.6 Initial temperature of HCl solution (C) 20.5 20.9 Initial temperature of NaOH solution (C) 20.7 20.5 Temperature of solution every 0.25 min/15 sec (C) 0.25 min 23.2 23.2 0.50 min 26.2 26.1 0.75 min 28.7 29.0 1.00 min 31.7 31.7 1.25 min 34.2 34.1 1.50 min 34.2 34.1 1.75 min 33.9 33.4 2.00 min 33.5 32.4 2.25 min 32.5 0.0 2.50 min 0.0 0.0 2.75 min 0.0 0.0 3.00 min 0.0 0.0 3.25 min 0.0 0.0 3.50 min 0.0 0.0 3.75 min 0.0 0.0 4.00 min 0.0 0.0 Maximum temperature (C) (To be used in calculations) 34.2 34.1 Time-temperatue data for Part B Run 1 Run 2 Mass of weighing paper (9) 0.3555 0.3361 Mass of weighing paper and Mg (9) 0.8716 0.8462 Molarity of HCl solution (M) 2.054 2.054 Volume of HCl solution (mL) 50.4 49.5 Volume of water added to HCl solution (mL) 49.7 48.0 Initial temperature of HCl solution (C) 20.5 20.9 Temperature of solution every 0.25 min/15 sec (C) 0.25 min 22.6 23.3 0.50 min 24.9 25.5 0.75 min 27.2 27.6 1.00 min 29.0 29.5 1.25 min 31.4 31.6 1.50 min 33.6 34.2 1.75 min 35.7 36.4 2.00 min 38.0 38.1 2.25 min 40.1 40.4 2.50 min 42.5 42.9 2.75 min 42.5 42.9 3.00 min 41.7 42.0 3.25 min 40.4 40.8 3.50 min 38.3 0.0 3.75 min 0.0 0.0 4.00 min 0.0 0.0 Maximum temperature (C) (To be used in calculations) 42.5 42.9 (10.5pts) Part A: Determining the Heat Capacity of the Calorimeter Complete the table below. It may be helpful to refer to the pre-lab questions and sample calculations in the lab manual. Table view List view Part A: Heat capacity calculations Run 1 Run 2 Initial temperature of HCl solution (C) 20.5 20.9 Initial temperature of NaOH solution (C) 20.7 20.5 Average initial temperature of HCl-NaOH solution (C) Maximum temperature of HCl-NaOH solution (C) 34.2 34.1 Maximum temperature change for reaction (C) Volume of HCl solution (mL) 50.4 50.8 Volume of NaOH solution (mL) 53.8 54.6 Total Volume of HCl plus NaOH solutions (mL) Mass of final NaCl solution (g) assume 1 M NaCl has a density of 1.04 g/mL Heat produced from HCl-NaOH reaction (J) assume the heat capacity of 1 M NaCl is 3.93 J/(g:C) Molarity of HCl solution (M) 2.054 2.054 Moles of H2O formed in reaction (mol) Heat expected from moles of H20 formed (J) assume 57,320 J per mole of H20 Heat absorbed by calorimeter (J) (heat expected-heat actually produced) Heat capacity of the calorimeter (J/C) (heat absorbed per degree of temperature change) (0.5pts) Average heat capacity of the calorimeter (J/C) Part B: Enthalpy change calculations Run 1 Run 2 Initial temperature of solution (C) 20.5 20.9 Maximum temperature of solution (C) 42.5 42.9 Maximum temperature change for reaction (C) Mass of weighing paper and Mg (9) 0.8716 0.8462 Mass of weighing paper (g) 0.3555 0.3361 Mass of Mg (9) Volume of HCl solution (mL) 50.4 49.5 Volume of water (mL) 49.7 48.0 Total mass of final solution (9) (mass of HCI, H20, and Mg) assume 2 M HCl has a density of 1.03 and water has a density of 1.00 g/mL Heat required to raise solution to maximum temperature (J) assume the solution has a heat capacity of 3.97 J/(gC) Heat required to raise calorimeter to maximum temperature (J) using the average heat capacity calculated in part A Total heat evolved by the reaction (J) Moles of Mg (mol) Heat evolved per mole of Mg (J/mol) Enthalpy change for reaction (J) (0.5pts) Average enthalpy (J) (1pts) 1. List and explain at least 2 specific sources of error in this experiment, and how those might have been avoided. Normal BIU X2 X2 = fx Tx (1 pts) 2. Is it important to have the temperatures of the HCl and NaOH solutions equal or very close to the same, at the beginning of part A? Justify your answer. Normal . BIU X2 X - == fx e Ei TX (1pts) 3. Suppose your sample of magnesium was contaminated with an equal weight of MgCl2. How would the contamination affect the change in enthalpy for the Mg-HCI reaction? Justify your answer. Normal . BIU X2 X -> = = fx D ili TX (1 pts) 4. Suppose you replaced the magnesium in Part B with an equal weight of sodium. How would this substitution affect the enthalpy of the reaction calculated in the experiment? Be specific and justify your answer. Normal . B I U X2 X === fo TX Part A Data Time-temperatue data for Part A Run 1 Run 2 Molarity of HCl solution (M) 2.054 2.054 Volume of HCl solution (mL) 50.4 50.8 Molarity of NaOH solution (M) 2.035 2.035 Volume of NaOH solution (mL) 53.8 54.6 Initial temperature of HCl solution (C) 20.5 20.9 Initial temperature of NaOH solution (C) 20.7 20.5 Temperature of solution every 0.25 min/15 sec (C) 0.25 min 23.2 23.2 0.50 min 26.2 26.1 0.75 min 28.7 29.0 1.00 min 31.7 31.7 1.25 min 34.2 34.1 1.50 min 34.2 34.1 1.75 min 33.9 33.4 2.00 min 33.5 32.4 2.25 min 32.5 0.0 2.50 min 0.0 0.0 2.75 min 0.0 0.0 3.00 min 0.0 0.0 3.25 min 0.0 0.0 3.50 min 0.0 0.0 3.75 min 0.0 0.0 4.00 min 0.0 0.0 Maximum temperature (C) (To be used in calculations) 34.2 34.1 Time-temperatue data for Part B Run 1 Run 2 Mass of weighing paper (9) 0.3555 0.3361 Mass of weighing paper and Mg (9) 0.8716 0.8462 Molarity of HCl solution (M) 2.054 2.054 Volume of HCl solution (mL) 50.4 49.5 Volume of water added to HCl solution (mL) 49.7 48.0 Initial temperature of HCl solution (C) 20.5 20.9 Temperature of solution every 0.25 min/15 sec (C) 0.25 min 22.6 23.3 0.50 min 24.9 25.5 0.75 min 27.2 27.6 1.00 min 29.0 29.5 1.25 min 31.4 31.6 1.50 min 33.6 34.2 1.75 min 35.7 36.4 2.00 min 38.0 38.1 2.25 min 40.1 40.4 2.50 min 42.5 42.9 2.75 min 42.5 42.9 3.00 min 41.7 42.0 3.25 min 40.4 40.8 3.50 min 38.3 0.0 3.75 min 0.0 0.0 4.00 min 0.0 0.0 Maximum temperature (C) (To be used in calculations) 42.5 42.9 (10.5pts) Part A: Determining the Heat Capacity of the Calorimeter Complete the table below. It may be helpful to refer to the pre-lab questions and sample calculations in the lab manual. Table view List view Part A: Heat capacity calculations Run 1 Run 2 Initial temperature of HCl solution (C) 20.5 20.9 Initial temperature of NaOH solution (C) 20.7 20.5 Average initial temperature of HCl-NaOH solution (C) Maximum temperature of HCl-NaOH solution (C) 34.2 34.1 Maximum temperature change for reaction (C) Volume of HCl solution (mL) 50.4 50.8 Volume of NaOH solution (mL) 53.8 54.6 Total Volume of HCl plus NaOH solutions (mL) Mass of final NaCl solution (g) assume 1 M NaCl has a density of 1.04 g/mL Heat produced from HCl-NaOH reaction (J) assume the heat capacity of 1 M NaCl is 3.93 J/(g:C) Molarity of HCl solution (M) 2.054 2.054 Moles of H2O formed in reaction (mol) Heat expected from moles of H20 formed (J) assume 57,320 J per mole of H20 Heat absorbed by calorimeter (J) (heat expected-heat actually produced) Heat capacity of the calorimeter (J/C) (heat absorbed per degree of temperature change) (0.5pts) Average heat capacity of the calorimeter (J/C) Part B: Enthalpy change calculations Run 1 Run 2 Initial temperature of solution (C) 20.5 20.9 Maximum temperature of solution (C) 42.5 42.9 Maximum temperature change for reaction (C) Mass of weighing paper and Mg (9) 0.8716 0.8462 Mass of weighing paper (g) 0.3555 0.3361 Mass of Mg (9) Volume of HCl solution (mL) 50.4 49.5 Volume of water (mL) 49.7 48.0 Total mass of final solution (9) (mass of HCI, H20, and Mg) assume 2 M HCl has a density of 1.03 and water has a density of 1.00 g/mL Heat required to raise solution to maximum temperature (J) assume the solution has a heat capacity of 3.97 J/(gC) Heat required to raise calorimeter to maximum temperature (J) using the average heat capacity calculated in part A Total heat evolved by the reaction (J) Moles of Mg (mol) Heat evolved per mole of Mg (J/mol) Enthalpy change for reaction (J) (0.5pts) Average enthalpy (J) (1pts) 1. List and explain at least 2 specific sources of error in this experiment, and how those might have been avoided. Normal BIU X2 X2 = fx Tx (1 pts) 2. Is it important to have the temperatures of the HCl and NaOH solutions equal or very close to the same, at the beginning of part A? Justify your answer. Normal . BIU X2 X - == fx e Ei TX (1pts) 3. Suppose your sample of magnesium was contaminated with an equal weight of MgCl2. How would the contamination affect the change in enthalpy for the Mg-HCI reaction? Justify your answer. Normal . BIU X2 X -> = = fx D ili TX (1 pts) 4. Suppose you replaced the magnesium in Part B with an equal weight of sodium. How would this substitution affect the enthalpy of the reaction calculated in the experiment? Be specific and justify your answer. Normal . B I U X2 X === fo TX
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


