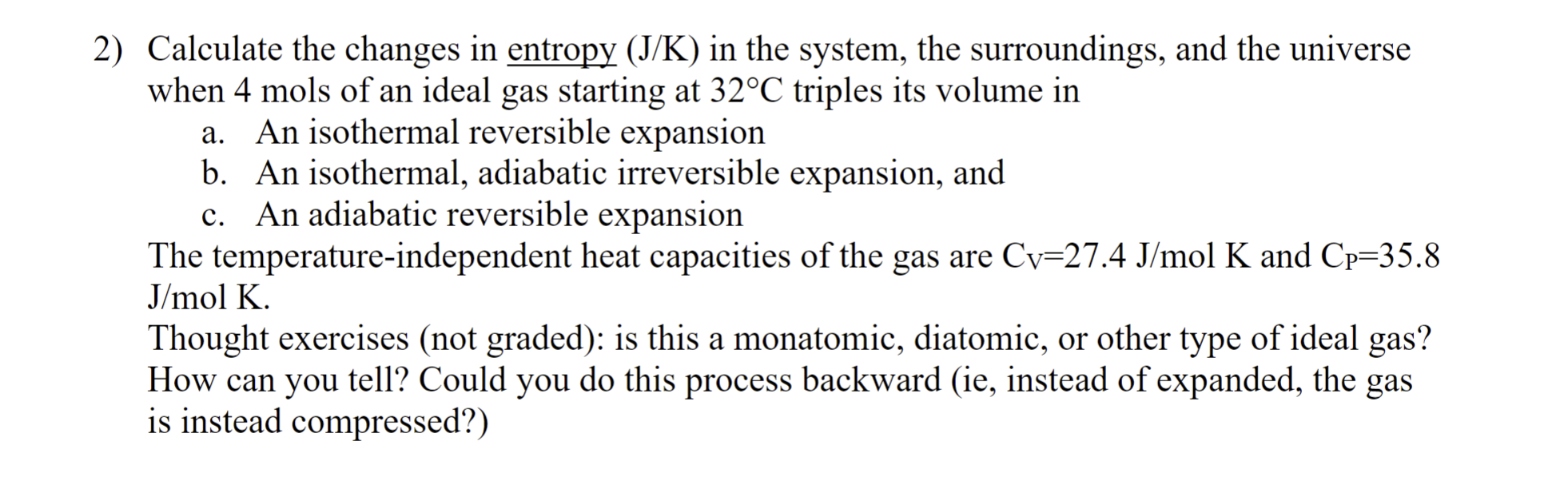
Question: Calculate the changes in entropy ( J K ) in the system, the surroundings, and the universe when 4 mols of an ideal gas starting
Calculate the changes in entropy in the system, the surroundings, and the universe
when mols of an ideal gas starting at triples its volume in
a An isothermal reversible expansion
b An isothermal, adiabatic irreversible expansion, and
c An adiabatic reversible expansion
The temperatureindependent heat capacities of the gas are olK and
olK.
d is this a monatomic, diatomic, or other type of ideal gas?
How can you tell? Could you do this process backward ie instead of expanded, the gas
is instead compressed?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


